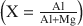Hydrotalcites of high aluminum content have been synthesized from aluminate liquors of varying composition and activated magnesia obtained by calcination of hydroxide or hydroxycarbonate precursors. Lattice parameter measurements and chemical analyses of 21 synthetic hydrotalcites show that the aluminum substitution $$\left( {\rm{X = \frac{{Al}}{{Al + Mg}}}} \right)$$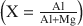 for most of the products is about 0.35, which is at the maximum experimentally-observed limit of solid solubility. Pillared hydrotalcites were also prepared by molybdate, Chromate, and silicate anion replacement. A maximum distance of 10.4 Å between the brucite-like layers was observed for the Mo7O246− intercalated material.
for most of the products is about 0.35, which is at the maximum experimentally-observed limit of solid solubility. Pillared hydrotalcites were also prepared by molybdate, Chromate, and silicate anion replacement. A maximum distance of 10.4 Å between the brucite-like layers was observed for the Mo7O246− intercalated material.