To identify the far-infrared (FIR) absorption bands related to K cations in micas, spectra of muscovite, phlogopite, and biotite were compared with the spectra of pyrophyllite and talc, which have no compensating cations, and to the spectra of micas in which K is substituted by mono- and divalent cations. Dichroic experiments using single crystals of these micas showed that some of the bands or components related to K have a strong in-plane and out-of-plane dichroic character. These experimental data led to the assignment of the FIR bands at 110, 91, and 83 cm−1 for muscovite, phlogopite, and biotite, respectively, which have no in-plane and out-of-plane dichroic character, to the vibration of the double ring of oxygen atoms that constitutes the cage in which K is located (mode III).
Bands at 146, 136, and 152 cm−1 for muscovite, phlogopite, and biotite, respectively, which have a strong out-of-plane dichroic character, were assigned to the out-of-plane vibrations of K atoms (mode IV). Some of the components at 190, 158–153, and 145–124 cm−1 for muscovite, phlogopite, and biotite, respectively, which have a strong in-plane dichroic character, are possibly related to the in-plane vibrations of K atoms (modes I and II). The 165-cm−1 band of muscovite is a lattice mode of vibration. Frequencies of modes III and IV of the compensating cations of micas in which K was substituted by mono- and divalent cations exchanged as a function of 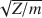 where Z is the charge and m the mass of the cation. Modes III and IV were well resolved and very sensitive to the crystallochemical properties of the structure (di- or trioctahedral character, Fe content, etc.). FIR spectroscopy may therefore be an important tool that uses compensating cations as probes to study the interactions between the cations and the structure of mica minerals.
where Z is the charge and m the mass of the cation. Modes III and IV were well resolved and very sensitive to the crystallochemical properties of the structure (di- or trioctahedral character, Fe content, etc.). FIR spectroscopy may therefore be an important tool that uses compensating cations as probes to study the interactions between the cations and the structure of mica minerals.