A GABA- or glycine-induced increase in Cl− permeability can produce either a depolarization or hyperpolarization, depending on the Cl− equilibrium potential. It has been shown that retinal neurons express the chloride cotransporters, Na-K-2Cl (NKCC) and K-Cl (KCC), the primary molecular mechanisms that control the intracellular Cl− concentration. We thus studied (1) the localization of these cotransporters in the fish retina, and (2) how suppression of cotransporter activity in the fish retina affects function. Specific antibodies against NKCC and KCC2 revealed that both cotransporters were expressed in the outer and inner plexiform layers, and colocalized in many putative amacrine cells and in cells of the ganglion cell layer. However, the somata of putative horizontal cells displayed only NKCC immunoreactivity and many bipolar cells were only immunopositive for KCC2. In the outer retina, application of bumetanide, a specific inhibitor of NKCC activity, (1) increased the steady-state extracellular concentration of K+ ([K+]o) and enhanced the light-induced decrease in the [K+]o, (2) increased the sPIII photoreceptor-dependent component of the ERG, and (3) reduced the extracellular space volume. In contrast, in the outer retina, application of furosemide, a specific inhibitor of KCC activity, decreased sPIII and the light-induced reduction in [K+]o, but had little effect on steady-state [K+]o. In the inner retina, bumetanide increased the sustained component of the light-induced increase in [K+]o. These findings thus indicate that NKCC and KCC2 control the [K+]o and extracellular space volume in the retina in addition to regulating GABA- and glycine-mediated synaptic transmission. In addition, the anatomical and electrophysiological results together suggest that all of the major neuronal types in the fish retina are influenced by chloride cotransporter activity.

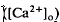 in the intact cat eye using ion-sensitive double-barreled microelectrodes. Two prominent changes in Ca
in the intact cat eye using ion-sensitive double-barreled microelectrodes. Two prominent changes in Ca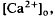 accompanied by brief ON and OFF transients, which was maximal in the inner plexiform layer and was not further studied. There was an unexpected sustained light-evoked
accompanied by brief ON and OFF transients, which was maximal in the inner plexiform layer and was not further studied. There was an unexpected sustained light-evoked 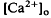 of relatively rapid onset and offset, which was maximal in the distalmost region of the subretinal space (SRS).
of relatively rapid onset and offset, which was maximal in the distalmost region of the subretinal space (SRS). 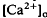 in the SRS was 1.0 mM higher than in the vitreous humor during dark adaptation and this transretinal gradient disappeared during rod-saturating illumination. After correcting for the light-evoked increase in the volume of the SRS, an increase in the total Ca
in the SRS was 1.0 mM higher than in the vitreous humor during dark adaptation and this transretinal gradient disappeared during rod-saturating illumination. After correcting for the light-evoked increase in the volume of the SRS, an increase in the total Ca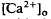 changes, we used the diffusion model described in the accompanying paper (Li et al., 1994
changes, we used the diffusion model described in the accompanying paper (Li et al., 1994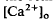 around photoreceptors, which persists during illumination and reduces a transretinal Ca
around photoreceptors, which persists during illumination and reduces a transretinal Ca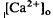 decrease remains unexplained. These steady-state shifts in
decrease remains unexplained. These steady-state shifts in 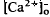 should have significant effects on photoreceptor function, especially adaptation.
should have significant effects on photoreceptor function, especially adaptation.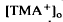 , which was maximal in amplitude in the most distal portion of the space surrounding photoreceptors, the subretinal space. The light-evoked decrease in
, which was maximal in amplitude in the most distal portion of the space surrounding photoreceptors, the subretinal space. The light-evoked decrease in 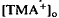 was considerably slower and of a different overall time course than the light-evoked decrease in
was considerably slower and of a different overall time course than the light-evoked decrease in 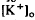 , also recorded in the subretinal space.
, also recorded in the subretinal space. 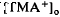 decreased to a peak at 38 s after the onset of illumination, then slowly recovered towards the baseline, and transiently increased following the offset of illumination. It resembled the light-evoked
decreased to a peak at 38 s after the onset of illumination, then slowly recovered towards the baseline, and transiently increased following the offset of illumination. It resembled the light-evoked 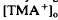 decreases previously recorded in the
decreases previously recorded in the 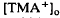 decrease is considered to originate from a light-evoked increase in the volume of the subretinal space (or subretinal hydration). A mathematical model accounting for
decrease is considered to originate from a light-evoked increase in the volume of the subretinal space (or subretinal hydration). A mathematical model accounting for 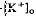 diffusion predicted that the volume increase underlying the response was 63% on average and could be as large as 95% and last for minutes. The estimated volume increase was then used to examine its effect on K
diffusion predicted that the volume increase underlying the response was 63% on average and could be as large as 95% and last for minutes. The estimated volume increase was then used to examine its effect on K