Mixed FeIIFeIII hydroxides, commonly referred to as ‘green rusts’ (GRs), are important reactive phases in both man-made and natural geochemical systems. Determinations of the standard Gibbs energy of formation of GRs are needed to understand and predict the occurrence and possible reactions of GRs in these systems. Slow acid titration of crystalline green rust sulfate (\$\end{document}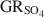 ) with the formation of magnetite was used as a novel method to determine the standard Gibbs energy of formation of \$\end{document}
) with the formation of magnetite was used as a novel method to determine the standard Gibbs energy of formation of \$\end{document}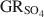 , \$\end{document}
, \$\end{document}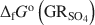 . Aqueous suspensions of \$\end{document}
. Aqueous suspensions of \$\end{document}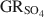 , with pH slightly >8, were titrated slowly with 1 M H2SO4 until pH = 3 under strict anoxic conditions. Powder X-ray diffraction and Mössbauer analysis revealed that magnetite was the only solid phase formed during the initial part of the titration, where the equilibrium pH was maintained above 7.0. The ratio of Fe2+ release to consumption of protons confirmed the stoichiometry of dissolution of \$\end{document}
, with pH slightly >8, were titrated slowly with 1 M H2SO4 until pH = 3 under strict anoxic conditions. Powder X-ray diffraction and Mössbauer analysis revealed that magnetite was the only solid phase formed during the initial part of the titration, where the equilibrium pH was maintained above 7.0. The ratio of Fe2+ release to consumption of protons confirmed the stoichiometry of dissolution of \$\end{document}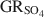 and the formation of magnetite at equilibrium conditions. The estimate of the absolute value of \$\end{document}
and the formation of magnetite at equilibrium conditions. The estimate of the absolute value of \$\end{document}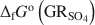 was −3819.43±6.44 kJ mol−1 + y × [ΔfGo(H2O(1))], where y is the number of interlayer water molecules per formula unit. The logarithm of the solubility product, log Ksp, was estimated to be −139.2±4.8 and is invariable with y. Using the new value for \$\end{document}
was −3819.43±6.44 kJ mol−1 + y × [ΔfGo(H2O(1))], where y is the number of interlayer water molecules per formula unit. The logarithm of the solubility product, log Ksp, was estimated to be −139.2±4.8 and is invariable with y. Using the new value for \$\end{document}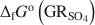 , the reduction potentials of several \$\end{document}
, the reduction potentials of several \$\end{document}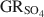 -Fe oxide couples were evaluated, with the \$\end{document}
-Fe oxide couples were evaluated, with the \$\end{document}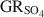 -magnetite half cell showing the smallest redox potential at pH 7 and free ion activities of 10−3.
-magnetite half cell showing the smallest redox potential at pH 7 and free ion activities of 10−3.