High-charge nontronites were synthesized at 75, 90, 100, 110, 125, and 150°C from a silicoferrous starting gel with Si2FeNa2O6.nH2O composition. This gel was oxidized in contact with air and then hydrothermally treated, for a period of 4 weeks, under equilibrium water pressure. The synthesized nontronites were similar to each other, regardless of the synthesis temperature. Their structural formula, obtained from chemical analysis, X-ray diffraction (XRD), and Fourier transform infrared (FTIR), Mössbauer, and X-ray absorption fine structure spectroscopies is: $\left( {{\rm{S}}{{\rm{i}}_{3.25}}{\rm{Fe}}_{0.75}^{3 + }} \right){\rm{Fe}}_2^{3 + }{{\rm{O}}_{10}}{\left( {{\rm{OH}}} \right)_2}{\rm{N}}{{\rm{a}}_{0.75}}$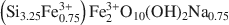 . A strictly ferric end-member of the nontronite series was therefore synthesized for the first time. The uncommon chemistry of the synthesized nontronites, notably the high level of Fe-for-Si substitution, induced particular XRD, FTIR, and differential thermal analysis-thermogravimetric analysis data. The ethylene glycol expandability of the synthetic nontronites was linked to their crystallinity and depended on the nature of the interlayer cation, moving from smectite to vermiculite-like behavior. As the synthesis temperature increased, the crystallinity of the synthesized clays increased. The nontronite obtained at 150°C had the ‘best crystallinity’, which cannot be improved by increasing synthesis time or temperature.
. A strictly ferric end-member of the nontronite series was therefore synthesized for the first time. The uncommon chemistry of the synthesized nontronites, notably the high level of Fe-for-Si substitution, induced particular XRD, FTIR, and differential thermal analysis-thermogravimetric analysis data. The ethylene glycol expandability of the synthetic nontronites was linked to their crystallinity and depended on the nature of the interlayer cation, moving from smectite to vermiculite-like behavior. As the synthesis temperature increased, the crystallinity of the synthesized clays increased. The nontronite obtained at 150°C had the ‘best crystallinity’, which cannot be improved by increasing synthesis time or temperature.