Over several decades, a wealth of literature has been devoted to correlations between the chemistries of phyllosilicates and their crystallographic unit-cell parameter values. The c parameter is currently used because of its relation to the layer-to-layer distance, characteristic of the various families of phyllosilicates. The b parameter is also of interest because it allows measurement of the layer lateral dimensions and inherent structural adjustments. This unit-cell distance can be extracted from X-ray diffraction traces from the (06ℓ;33ℓ) diffraction region and by attributing the main diffraction peak observed to a 060 reflection, leading to the relationship b = 6.d(060). The aim of this paper is to revisit the relationships between the b value (or equivalent) of the phyllosilicate (i.e. TO, TOT and TOTO) or hydroxide (i.e. hydroxide, oxyhydroxide and layered double hydroxide) families and the layer chemistry based on a mean ionic radius R of octahedral cations, calculated as $R = \mathop \sum \nolimits_{i = 1}^n ( {r_i.x_i} )$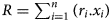 , where ri is the ionic radius of the octahedral cation i and xi is its molar fraction over n types of octahedral cations ($\mathop \sum \nolimits_{i = 1}^n ( {x_i} ) = 1$
, where ri is the ionic radius of the octahedral cation i and xi is its molar fraction over n types of octahedral cations ($\mathop \sum \nolimits_{i = 1}^n ( {x_i} ) = 1$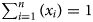 ). The data were collected from the literature and involved both natural and synthetic samples with both dioctahedral and trioctahedral structures of the octahedral sheet. The results showed that b values can be linked strongly to R, leading to suitable linear regressions for all of the studied structures. All correlations were found to be applicable irrespective of the di- or trioctahedral nature of the octahedral sheet, and these are discussed in light of (1) the lateral dimension of the octahedral sheet and (2) the dimensional misfit between the tetrahedral and octahedral sheets. For hydroxide families, all data can be gathered on a single b vs R correlation line, and the dimensional properties of the octahedral sheet can be interpreted simply based on an oxygen–cation–oxygen mean distance. For TO structures, two general b vs R correlation trends were reported, and these were assigned to two adjustment mechanisms corresponding to distinct types of tetrahedral and octahedral distortions. For the mica TOT family, two main trends were also reported, whereas the use of the synthetic mica series allowed us to demonstrate that the obtained scattering of data was mainly driven by the presence of multiple limited solid solutions. Such chemical complexity was also noted for smectites, especially regarding the tetrahedral composition and associated variability in layer charge. This variability made it difficult to propose a general regression correlating b to R values for smectites, although the regression obtained for neutral TOT layers can apply as a first-order relation. Finally, a single general b vs R correlation was obtained for chlorites, and the observed slope of the regression was interpreted according to the role played by the isolated hydroxide sheet on the evolution of the lateral dimension of the structures.
). The data were collected from the literature and involved both natural and synthetic samples with both dioctahedral and trioctahedral structures of the octahedral sheet. The results showed that b values can be linked strongly to R, leading to suitable linear regressions for all of the studied structures. All correlations were found to be applicable irrespective of the di- or trioctahedral nature of the octahedral sheet, and these are discussed in light of (1) the lateral dimension of the octahedral sheet and (2) the dimensional misfit between the tetrahedral and octahedral sheets. For hydroxide families, all data can be gathered on a single b vs R correlation line, and the dimensional properties of the octahedral sheet can be interpreted simply based on an oxygen–cation–oxygen mean distance. For TO structures, two general b vs R correlation trends were reported, and these were assigned to two adjustment mechanisms corresponding to distinct types of tetrahedral and octahedral distortions. For the mica TOT family, two main trends were also reported, whereas the use of the synthetic mica series allowed us to demonstrate that the obtained scattering of data was mainly driven by the presence of multiple limited solid solutions. Such chemical complexity was also noted for smectites, especially regarding the tetrahedral composition and associated variability in layer charge. This variability made it difficult to propose a general regression correlating b to R values for smectites, although the regression obtained for neutral TOT layers can apply as a first-order relation. Finally, a single general b vs R correlation was obtained for chlorites, and the observed slope of the regression was interpreted according to the role played by the isolated hydroxide sheet on the evolution of the lateral dimension of the structures.