The kinetics and reaction mechanisms of chlorite breakdown have been studied in a series of experiments at conditions similar to those achieved during contact metamorphism (T = 600-725°C P = 1 kbar). Cores of chlorite schist were used as starting material in order to simulate natural metamorphic systems and preserve reaction textures. Reaction products were analysed by electron microprobe, scanning- and transmission-electron microscopy (SEM, TEM). Although the texture of the original chlorite was preserved in experiments run below 680°C talc had replaced chlorite. Olivine and spinel formed along grain boundaries, indicating long-range diffusion of aluminium. Above 680°C the chlorite was replaced by patches of disordered, aluminous pyroxene. Olivine and spinel grew both within the pyroxene and along what are believed to be former chlorite grain-boundaries. Reactions relevant to the observed textures and assemblages are:
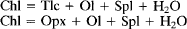
Thermodynamic calculations show that both of these reactions are metastable in the FeO-MgO-Al2O3-SiO2-H2O system in the P-T range of our experiments. In addition, previous experimental studies and our calculations indicate that the stable reaction is:
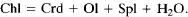
The absence of cordierite in the run products, and the formation of talc and orthopyroxene while thermodynamically metastable, show that the ease of nucleation of these phases controlled the reaction mechanisms in the early stages.