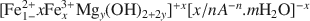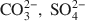Fougerite (IMA 2003-057) is a mixed M(II)-M(III) hydroxysalt of the green rust group, where M(II) can be Fe or Mg, and M(III) is Fe. The general structural formula is: 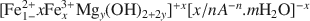 where A is the interlayer anion and n its valency, with 1/4 ≼ x/(1+y) ≼ 1/3 and m ≼ (1−x+y). The structure of green rusts and parent minerals can accommodate a variety of anions, such as OH−, Cl−, ${\rm{CO}}_3^{2 - },\;{\rm{SO}}_4^{2 - }$
where A is the interlayer anion and n its valency, with 1/4 ≼ x/(1+y) ≼ 1/3 and m ≼ (1−x+y). The structure of green rusts and parent minerals can accommodate a variety of anions, such as OH−, Cl−, ${\rm{CO}}_3^{2 - },\;{\rm{SO}}_4^{2 - }$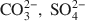 . The structure of the mineral was studied by Mössbauer, Raman and X-ray absorption spectroscopies (XAS) at the FeK edge. Mössbauer spectra of the mineral obtained at 78 K are best fitted with four doublets: D1 and D2 due to Fe2+ (isomer shift δ ≈ 1.27 and 1.25 mm s−1, quadrupole splitting ΔEQ ≈ 2.86 and 2.48 mm s−1, respectively) and D3 and D4 due to Fe3+ (δ ≈ 0.46 mm s−1, ΔEQ ≈ 0.48 and 0.97 mm s−1, respectively). Microprobe Raman spectra obtained with a laser at 514.53 nm show the characteristic bands of synthetic green rusts at 427 and 518 cm−1. X-ray absorption spectroscopy shows that Mg is present in the mineral in addition to Fe, that the space group is and the lattice parameter a ≈ 0.30–0.32 nm. The mineral forms by partial oxidation and hydrolysis of aqueous Fe2+, to give small crystals (400–500 nm) in the form of hexagonal plates. The mineral is unstable in air and transforms to lepidocrocite or goethite. The name is for the locality of the occurrence, a forested Gleysol near Fougères, Brittany, France. Its characteristic blue-green color (5BG6/1 in the Munsell system) has long been used as a universal criterion in soil classification to identify Gleysols. From a thermodynamic model of soil-solution equilibria, it was proposed that for the eponymous mineral, Fougères-fougerite, OH− may be the interlayer anion. In other environments, the interlayer anion may be different, and other varieties of fougerite may exist. Fougerite plays a key role in the pathways of formation of Fe oxides.
. The structure of the mineral was studied by Mössbauer, Raman and X-ray absorption spectroscopies (XAS) at the FeK edge. Mössbauer spectra of the mineral obtained at 78 K are best fitted with four doublets: D1 and D2 due to Fe2+ (isomer shift δ ≈ 1.27 and 1.25 mm s−1, quadrupole splitting ΔEQ ≈ 2.86 and 2.48 mm s−1, respectively) and D3 and D4 due to Fe3+ (δ ≈ 0.46 mm s−1, ΔEQ ≈ 0.48 and 0.97 mm s−1, respectively). Microprobe Raman spectra obtained with a laser at 514.53 nm show the characteristic bands of synthetic green rusts at 427 and 518 cm−1. X-ray absorption spectroscopy shows that Mg is present in the mineral in addition to Fe, that the space group is and the lattice parameter a ≈ 0.30–0.32 nm. The mineral forms by partial oxidation and hydrolysis of aqueous Fe2+, to give small crystals (400–500 nm) in the form of hexagonal plates. The mineral is unstable in air and transforms to lepidocrocite or goethite. The name is for the locality of the occurrence, a forested Gleysol near Fougères, Brittany, France. Its characteristic blue-green color (5BG6/1 in the Munsell system) has long been used as a universal criterion in soil classification to identify Gleysols. From a thermodynamic model of soil-solution equilibria, it was proposed that for the eponymous mineral, Fougères-fougerite, OH− may be the interlayer anion. In other environments, the interlayer anion may be different, and other varieties of fougerite may exist. Fougerite plays a key role in the pathways of formation of Fe oxides.