The previously unknown crystal structure of the basic lead nitrate Pb2(OH)3(NO3) has been determined using single-crystal X-ray diffraction data (Mo-Kα radiation, CCD area detector). The compound is orthorhombic, space group Immm, with a = 8.314(2), b = 8.545(2), c = 17.210(3) Å (R1 = 2.78% for 940 ‘observed’ reflections with Fo > 4σ(Fo)). The layered structure contains a previously unknown cuboid [Pb8(OH)12]4+ cluster and NO3 groups. The study used crystals formed by anthropogenic processes on a medieval mine dump, probably involving black gunpowder used in the blasting of ore. Pb2(OH)3(NO3) is associated with a second nitrate, Pb13O8(OH)6(NO3)4, which was previously designated as “Pb3O3(OH)4(NO3)2” or “Pb3(OH)5(NO3)”. It is rhombohedral, space group 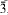 , with a = 10.263(1), c = 25.454(5) Å, and a structure solution is in complete agreement with an independent single-crystal study by Li et al. (2001). Probable hydrogen bonds in Pb2(OH)3(NO3) are indicated. Reported data on
, with a = 10.263(1), c = 25.454(5) Å, and a structure solution is in complete agreement with an independent single-crystal study by Li et al. (2001). Probable hydrogen bonds in Pb2(OH)3(NO3) are indicated. Reported data on 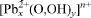 clusters and lead hydroxide and oxide nitrates are summarized and discussed critically. The probable conditions of formation of the studied samples are evaluated.
clusters and lead hydroxide and oxide nitrates are summarized and discussed critically. The probable conditions of formation of the studied samples are evaluated.