Tetrahedrite fahlores from the Coeur d'Alene mining district (Idaho) have been found to be enriched in Ag by the Ag–Cu exchange reaction
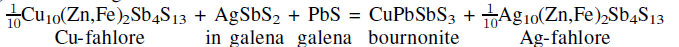
which occurred during cooling following galena mineralization. This solid–state reaction resulted in quantitative removal of Ag (in a AgSbS2 component) from galena and development of bournonite coronas on fahlore grains. The reaction produced a distinct population of high-Ag fahlores found in galena-rich samples and accounts for all of the bournonite mineralization. The most argentian of these high-Ag fahlores examined in this study (molar Ag/(Ag+Cu) = 0.303±0.011 and 0.336±0.011) are found in samples which achieved saturation with respect to other Ag-sulfosalts, namely pyrargyrite and polybasite and diaphorite, respectively. Multiple lines of evidence for the Ag-Cu exchange reaction are presented in this paper which applies mass-balance constraints and a thermodynamic database for sulfides/sulfosalts to microprobe analyses and textural observations, and to bulk production data. This solid-state reaction may explain why previous district studies have been unable to demonstrate convincingly primary fahlore zoning. Based on the Ag/(Ag+Cu) of fahlores coexisting with other Agsulfosalts and Fe-Zn partitioning between fahlore and sphalerite, we estimate that fahlore compositions were frozen in by 175°C. Examining the composition data for other Ag tetrahedrite fahlores found in the Coeur d'Alene district and elsewhere, we conclude that the thermodynamic database provides an accurate description of phase equilibria, except possibly for very Fe-rich systems where further studies of the Fe-Zn partitioning between fahlore and Fe-rich (Zn,Fe)S phases and of the thermodynamics of Fe-rich (Zn,Fe)S phases are warranted.