1.1 Organs
Plants are essentially modular organisms; each individual plant consists of distinct but connected organs. In their turn, the organs are composed of cells, which are mostly grouped into tissues. Vegetative organs support photosynthesis and plant growth, and reproductive organs enable sexual reproduction. In seed plants, the primary vegetative organs are the root, stem and leaf (Figure 1.1). Roots and stems have well-defined growing points at their apices, but the leaves are determinate lateral organs that stop growing when they reach a particular size and shape. When a seed germinates, the seed coat (testa) is ruptured and the embryonic structures emerge from opposite poles of the embryo: a seedling root (radicle) grows downwards from the root apex and a seedling axis (hypocotyl) bears the first leaves (cotyledons) and the shoot apex, which ultimately develops new foliage leaves.
1.2 Cells and cell walls
Plant cells consist of a living protoplast contained within a protein-rich plasma membrane, which is itself enclosed by a cell wall. During the cell-division cycle, cells undergo a series of phases that are broadly grouped as interphase, nuclear division (mitosis or meiosis) and cytokinesis (cleavage of the cytoplasm and cell-wall formation). During mitosis, nuclear division occurs first, followed by progressive deposition of membranes in the cytoplasm into a cell plate that is located in the equatorial zone between the two daughter nucleiReference Sylvester138. The cell plate extends to join the cell wall, thus depositing a new wall.
The primary cell wall consists mostly of carbohydrates: microfibrils of cellulose and hemicelluloses embedded in a matrix of pectins (Figure 1.2). Cell walls of adjacent cells are linked together by a pectin-rich middle lamellaReference Esau37, Reference Evert39. Following cell enlargement and elongation, a secondary cell wall is deposited on the inside surface of the primary wall. Secondary cell walls often appear layered and can contain deposits of complex organic polymers such as cutin, lignin and suberin. Cutin is the primary component of the plant cuticle, which covers the aerial epidermis (Section 1.8). Lignin provides strengthening and rigidity to sclerenchyma cells, especially in the secondary xylem, but also in fibres close to the vascular bundles in the stem and leaf. Suberin is a complex hydrophobic lipid that provides a protective water-resistant lipophilic barrier in periderm cells.
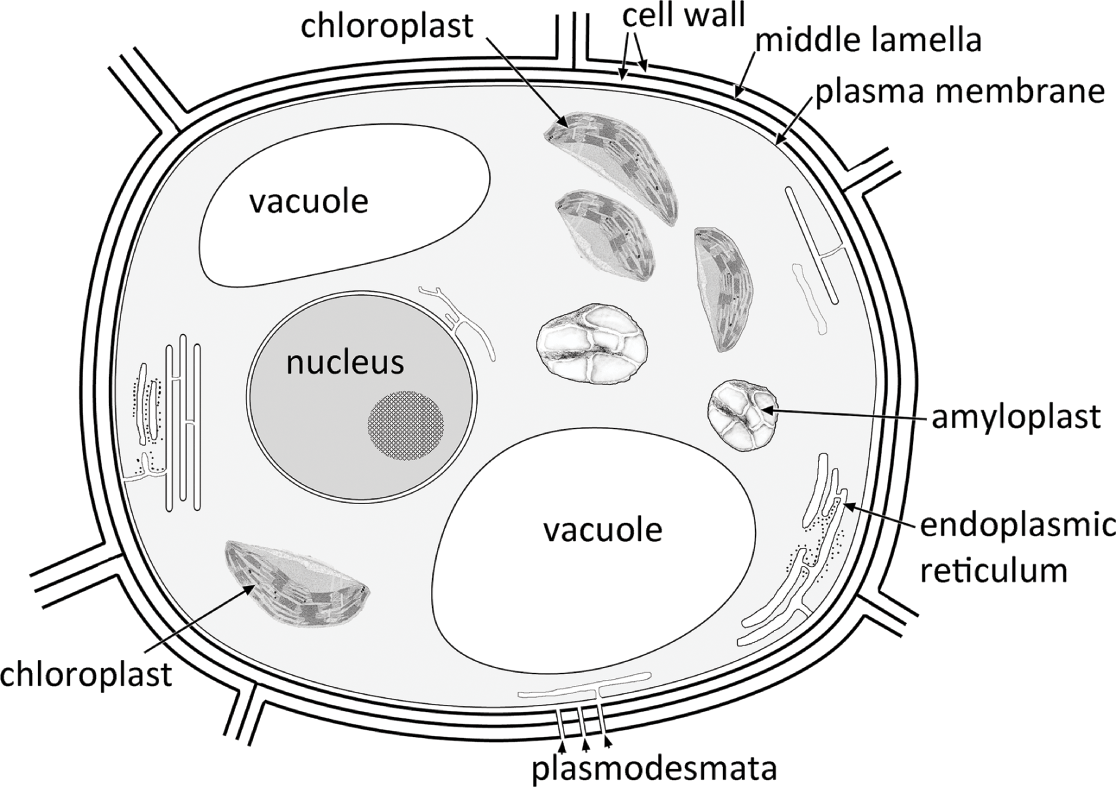
Figure 1.2 Diagram of a generalized plant cell
Primary pit fields are thin regions of the primary cell wall that correspond with similar regions in the walls of neighbouring cells. Pits have protoplasmic strands (plasmodesmata) passing through them, connecting the protoplasts of adjacent cells. The connected living protoplasts are collectively termed the symplast. Primary pit fields often persist as thin areas of the wall even after a secondary wall has been deposited. In simple pits, which occur on relatively non-specialized cells such as parenchyma, the pit cavity is of more or less uniform width. In bordered pits, which are present in tracheary elements, the secondary wall arches over the pit cavity so that the opening to the cavity is narrow and the outer rim of the primary pit field appears as a border around the pit opening when viewed through a light microscopeReference Choat, Cobb and Jansen18.
1.3 Cytoplasm, plastids and photosynthesis
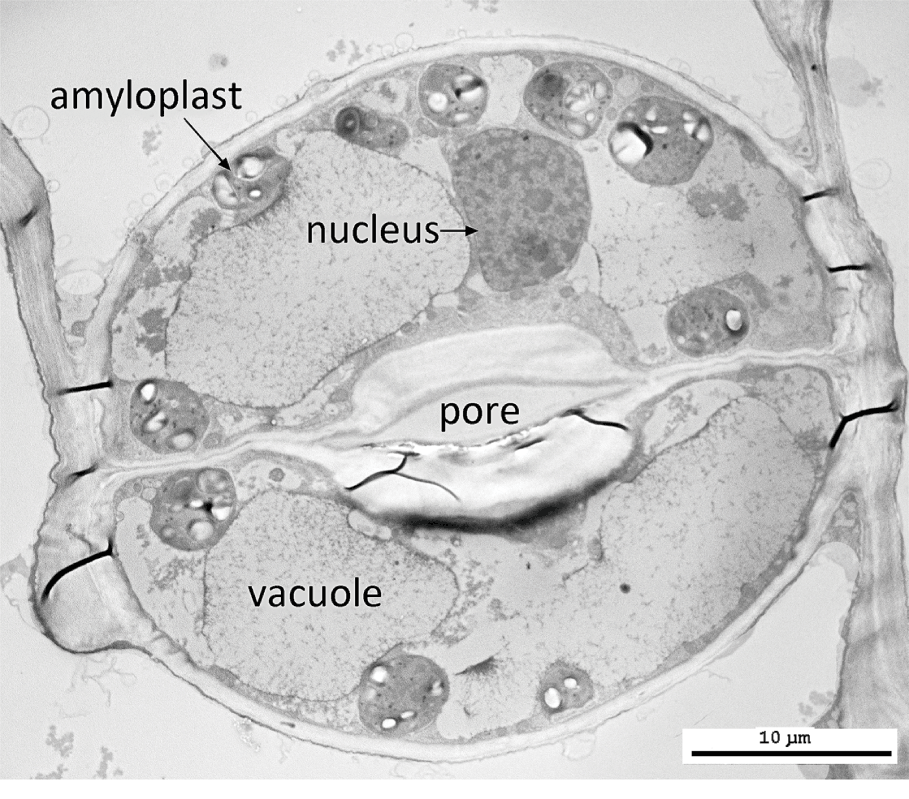
The living cell protoplast consists of cytoplasm that encloses a complex range of membrane-bound internal structures termed organelles; they include mitochondria, the nucleus, plastids and vacuoles, as well as small particles termed ribosomes and sometimes inorganic contents such as oil, starch grains or crystals (Figure 1.3).
Most plant cells possess a single nucleus that is bounded by a pair of membranes; the outer membrane is continuous with the endoplasmic reticulum. At interphase, one or more nucleoli can sometimes be distinguished, together with the uncondensed chromatin within the nuclear sap. During nuclear division, the chromatin becomes condensed into chromosomes that bear the hereditary information. Vacuoles are membrane-bound structures that contain a watery sap; they can vary considerably in size and shape during the life history of a cell. They can accumulate storage products and soluble pigments such as anthocyanins.
Plastids are large cell organelles that develop from proplastids. They each contain their own genome, which is much smaller than the nuclear genome and normally heritable via the maternal parent. Each plastid is bounded by a pair of membranes, and many contain a system of membranes termed thylakoids. Plastids such as amyloplasts, chloroplasts and chromoplasts play specialized roles within the cell. Amyloplasts are the source of starch grain production. Chromoplasts contain carotenoid pigments that produce some of the colours found in some plant organs, such as flower petals. Mitochondria – the sites of respiration within the cell – are smaller than plastids; they also contain their own heritable genetic material and are enclosed within a double-membrane system. Both plastids and mitochondria originated via endosymbiotic events at an early unicellular stage in plant evolutionReference Gould, Waller and McFadden50.
Chloroplasts are highly specialized plastids containing green chlorophyll proteins that absorb energy from sunlight. During photosynthesis, plants convert light energy into chemical energy in energy-storage molecules such as adenosine triphosphate (ATP), which they use to make carbohydrates from carbon dioxide (CO2) and water, releasing oxygen as a by-product. Chloroplasts occur in all green cells but are most abundant in the leaf mesophyll. In most plants, photosynthetic carbon reduction is achieved via a three-carbon compound (C3 cycle), but some plants capture inorganic carbon more effectively using CO2-concentrating mechanisms via a C4 cycle or Crassulacean acid metabolism (CAM)Reference Edwards31. Some C4 plants possess a distinctive leaf anatomy, termed Kranz anatomy (Section 4.9).
1.4 Inorganic cell inclusions
Many cells possess non-protoplasmic contents such as mucilage, oils, tannins, starch granules, calcium oxalate crystals and silica bodies. Both oil and mucilage are produced in isolated specialized secretory cells (idioblasts). Tannins are phenol derivatives that are widely distributed in plant cells; they are amorphous and appear yellow, red or brown in cells of sectioned material due to oxidation.
Starch is also widespread in plant tissues but especially common in storage tissues such as endosperm or in parenchyma adjacent to a nectary. Starch granules are formed in specialized plastids (amyloplasts). They often appear layered due to the successive deposition of concentric rings and possess characteristic shapes. The unusual and specialized starch grains present in laticifers in some species of Euphorbia possess highly characteristic elongated rod- or bone-like shapes compared with the more rounded starch grains of neighbouring parenchyma cellsReference Mahlberg85.
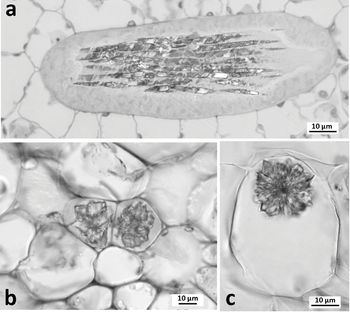
Calcium oxalate crystals (Figure 1.4) occur within crystal idioblasts; they can be distributed in almost every part of both vegetative and reproductive organs and are often located near veins, possibly reflecting transport of calcium through the xylem. They form within the vacuoles of actively growing cells and are usually associated with membrane chambers, lamellae, mucilage and fibrillar material. Contrasting crystal shapes can be highly characteristic of different plant families; for example, styloid crystals characterize the Iris family (Iridaceae). Common crystal types include solitary needle-like or rhomboidal crystals (styloids), bundles of needle-like crystals borne together in the same cell (raphides), aggregate crystalline structures that have precipitated around a nucleation site (druses) and numerous fine particles of crystal sand. In some woody eudicot species, crystals occur in the secondary phloem or secondary xylem; for example, crystal cells are common in the ray parenchyma of some woods (Chapter 2). Cystoliths are calcareous bodies that are mostly located in leaf epidermal cells (Figure 4.4).
Most plants deposit silica in at least some of their tissuesReference Prychid, Rudall and Gregory101. Some species accumulate silica in large quantities and deposit it as discrete bodies of solid silicon dioxide in the lumina of specific plant cells. Opaline silica bodies, commonly termed ‘phytoliths’, are a characteristic feature of some flowering plant groups. These groups include several commelinid monocots, notably the grass family PoaceaeReference Rudall, Prychid and Gregory118, in which silica bodies are almost exclusively restricted to the epidermis, and the palm family Arecaeae, in which they are primarily restricted to the vascular bundle sheath cells. Grass phytoliths occur in various shapes that can characterize different species, so they can have immense significance as diagnostic markers in studies of grassland palaeoecology (Figure 1.5). Silica bodies are often associated with sclerenchyma. In some woody eudicot species they can occur in the secondary xylem.
1.5 Meristems
Meristems are the growing points of the plant. They represent localized regions of thin-walled, tightly packed living cells that undergo frequent mitoses and often continue to divide indefinitely. Most of the plant body is differentiated at the meristems, though cells in other regions may also occasionally divide.
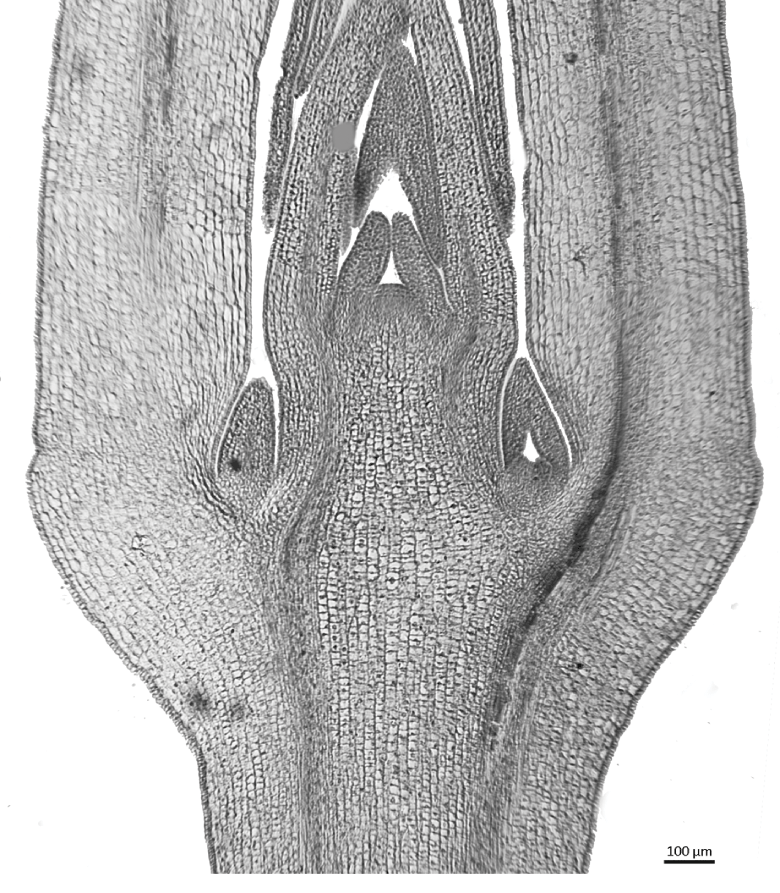
Apical meristems are located at the shoot apex (Figures 1.1, 2.1), where the primary stem, leaves and flowers differentiate, and at the root apex (Figures 3.1, 3.2), where primary root tissue is produced. In flowering plants, the shoot and root apical meristems are highly organized but differ from each other in many respects. Both shoot and root apical meristems contribute to extension growth and are self-renewing. The shoot apical meristem also initiates lateral organs (leaves) at its flanks in a regular nodal arrangement, each node bearing a single leaf, a pair of leaves or a whorl of leaves. Subsequent elongation of the shoot axis occurs at the stem internodes, either by diffuse cell divisions and growth throughout the youngest internodes (uninterrupted meristem) or in a restricted region, often at the base of the internode (intercalary meristem). Both intercalary and uninterrupted meristems represent growth in regions of differentiated tissues.
Lateral meristems are important for stem thickening growth; they include vascular cambium and the primary and secondary thickening meristems (Chapter 2). Lateral meristems occur in localized regions parallel to the long axis of a shoot or root, most commonly in the pericyclic zone, at the junction between vascular tissue and cortex. The phellogen (cork cambium) is a lateral meristem that occurs in the stem or root cortex, where it forms a protective corky layer (Figure 2.10); a phellogen can also develop in the region of a wound or at the point of leaf abscission.
Meristemoids are isolated and densely protoplasmic cells that reactivate embryonic activity to allow tissue differentiation. Typically, a meristemoid either itself undergoes unequal (asymmetric) cell division or is the smaller daughter cell that results from an asymmetric divisionReference Bünning and Avery16 (Figure 1.6). Asymmetric divisions are caused by cell polarization resulting from organized arrangements of actin filaments in the dense cytoplasm during cell plate alignmentReference Galatis and Apostolakos49. Examples of asymmetric cell divisions include formation of a root hair initial (trichoblast), microspore division into a larger vegetative cell and smaller generative cell, a protophloem mitosis to form a larger sieve tube element and smaller companion cell, and division of a protodermal cell into two cells of unequal sizes, the smaller of which is the guard-mother cell, a meristemoid that will divide symmetrically to form the paired guard cells of a stoma.

Figure 1.6 Amborella trichopoda (ANA-grade: Amborellaceae), developing leaf epidermis, highlighting a pair of cells that have recently undergone asymmetric mitosis to form a dense meristemoid and its larger sister cell (TEM). The meristemoid will form a guard-mother cell and will ultimately divide symmetrically to form a pair of guard cells. Scale = 2 µm
Other types of localized meristems occur in some species, in which differentiated plant cells can become de-differentiated and meristematic. One example of such cell-fate plasticity includes localized meristems that occur on the leaf margins of some succulent Crassulaceae; these meristematic cells can give rise to entire plantletsReference Steeves and Sussex131. Plants can also regenerate tissues and organs at the site of a wound by cellular proliferation on callus tissue, a process that is regulated by the plant hormones auxin and cytokinin. Callus cells formed from roots and from some aerial organs resemble the apices of lateral roots derived from the pericycleReference Lee and Seo81, Reference Sugimoto, Jiao and Meyerowitz137. Isolated callus tissue can be used in laboratory conditions to artificially grow a new plant using tissue culture methods.
1.6 Cell growth and expansion
Cells develop and expand in different ways depending partly on their location and surrounding tissue and partly on their cell-wall properties. Those in close contact with each other are initially glued together by a pectin-rich middle lamella and hence have a mutual influence on shape during early expansion. Parenchyma cells, which are largely thin walled and isodiametric, typically expand relatively evenly. Different rates of expansion of adjacent cells can result in the formation of lobes and intracellular air spaces, as in the spongy mesophyll of the leafReference Avery5. Adjacent tissues can also expand at different rates; for example, leaf epidermal cells continue to enlarge after the subepidermal mesophyll cells have ceased growth, influencing development of a substomatal cavity and anticlinal cell-wall undulations in abaxial epidermal cells. Epidermal cells, which require additional strength in their protective role, display differential growth between their anticlinal and periclinal wallsReference Jacques, Verbelen and Vissenberg64, Reference Kutschera78, Reference Sapala, Runions, Routier-Kierzkowska, Das Gupta, Hong, Hofhuis, Verger, Mosca, Li, Hay, Hamant, Roeder, Tsiantis, Prusinkiewicz and Smith126.
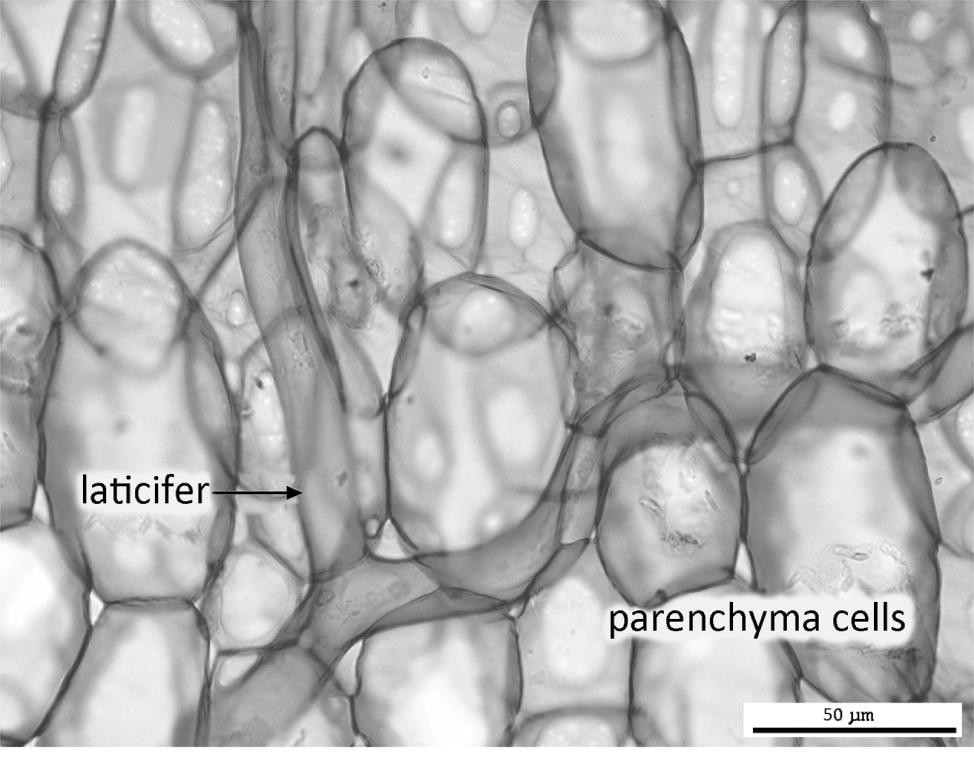
Some specialized cells are capable of apical intrusive growth (tip growth) that allows them to separate the middle lamella between adjacent cell walls and intrude between them, prior to the onset of cell-wall thickeningReference Jarvis, Briggs and Knox66, Reference Nezhad and Geitmann94. Fibres, which collectively confer tensile strength to tissues such as secondary xylem, extend axially at both ends by apical intrusive growth. Similarly, non-articulated laticifers (e.g. in Euphorbia) branch and ramify throughout the plant by apical growth (Figure 1.7). Other specialized cells capable of apical intrusive growth include root hairs, pollen tubes and star-shaped astrosclereids.
1.7 Tissues
Simple tissues, such as parenchyma, collenchyma and sclerenchyma, consist of regions of similar individual cells, often interspersed with isolated specialized cells (idioblastsReference Foster43) and secretory cells or canals. The bulk of the primary plant body (the ground tissue) consists of simple tissues. In contrast, complex tissues incorporate elements of several different simple cell and tissue types. Complex vascular tissues (phloem and xylem) can incorporate parenchyma, sclerenchyma and vascular cells. The epidermis is a dermal tissue that encloses the entire plant and is continuous between the various organs. Primary tissues are produced by an apical meristem, and secondary tissues are produced by lateral meristems such as the vascular cambium.
1.7.1 Parenchyma and other thin-walled cell types
The term parenchyma incorporates the relatively unspecialized cells that occur in both primary and secondary tissues of the plant body; it includes many cells with living contents that do not fit readily into other categories. Parenchyma cells are thin walled and often loosely packed together, leaving small air spaces between them where their walls are not in contact with each other. Individual parenchyma cells are polyhedral or amorphous, sometimes axially elongated or even lobed.
Chlorenchyma cells are green thin-walled cells that contain chloroplasts. In leaves (Chapter 4), chlorenchyma tissue is termed mesophyll. Mesophyll is often differentiated into two distinct zones: palisade mesophyll on the adaxial side of the lamina and spongy mesophyll on the abaxial side. Palisade mesophyll consists of rows of cells that are tightly packed together and anticlinally elongated; spongy mesophyll consists of cells of various shapes that are relatively loosely packed, with many intercellular air spaces.
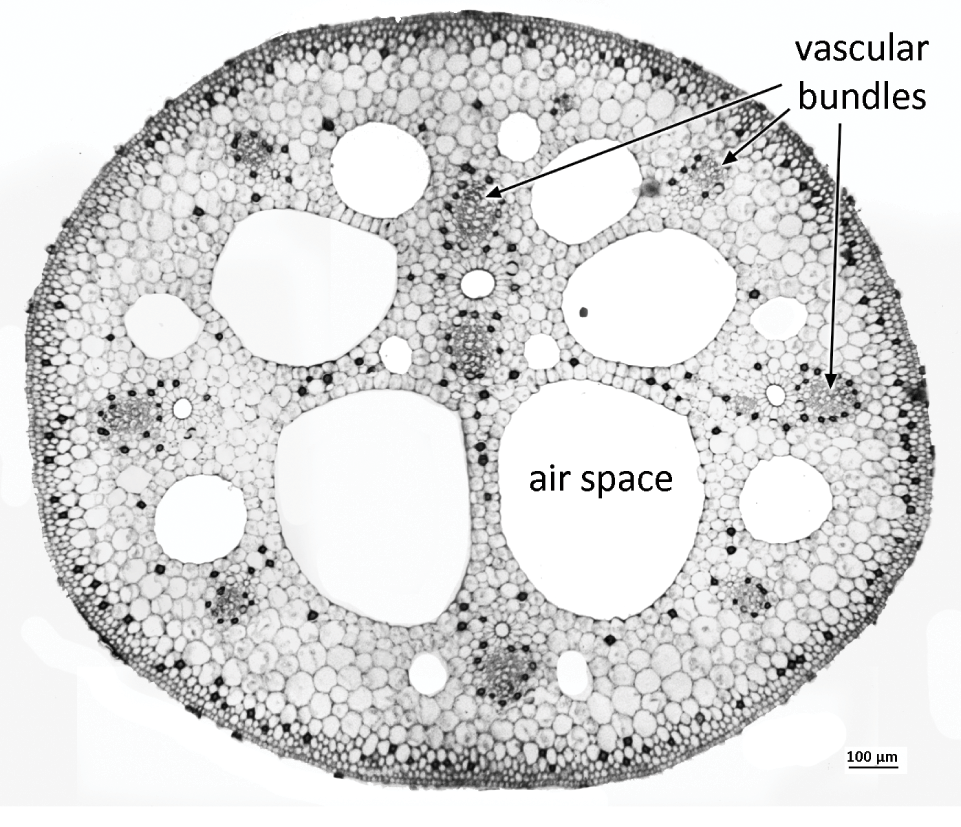
Aerenchyma is a specialized parenchymatous tissue that characterizes many aquatic plants. It represents a regular, well-developed system of cells interspersed with considerably enlarged intercellular air spaces that facilitate internal diffusion of gases (Figure 1.8). Contrasting developmental patterns of aerenchyma exist in different speciesReference Evans38. Lysigenous aerenchyma involves programmed cell death during development, leaving regular spaces (e.g. in the cortex of a maize root). In contrast, in schizogenous aerenchyma, which is relatively complex and more structured, the large air spaces are formed by differential growth of surrounding tissues. For example, in leaves, stems and roots of some aquatic plants (e.g. Hydrocharis), schizogenous aerenchyma is associated with a system of transverse septa or diaphragms that provide mechanical resistance to compression.
Transfer cells are specialized plant cells that facilitate transfer of soluble substances across tissue boundaries. They typically possess cell-wall ingrowths protruding into their protoplasts, thus increasing their surface area. They occur in companion cells in phloem (especially at the node of a stem), in root nodules, in the haustoria of parasitic plants and in the epidermis of water plantsReference Pate and Gunning98.
1.7.2 Thick-walled tissues: collenchyma and sclerenchyma
Collenchyma is a strengthening tissue that consists of groups of axially elongated, tightly packed cells with unevenly thickened walls. Collenchyma tissue typically occurs as primary ground tissue, often located in the angles of young stems, or in the leaf midrib or petiole. Collenchyma cells differ from fibres in that they often retain their living protoplasts and their cell walls are relatively unlignified, though they may ultimately become lignified as the tissue ages.
Sclerenchyma cells lack contents at maturity and possess evenly thickened walls that are usually lignified, with simple pits that are often slit-like. Sclerenchyma occurs in both primary and secondary tissues, either in groups or individually as idioblasts interspersed in other tissue types. Fibres are long, narrow sclerenchyma cells that are elongated along the long axis of the organ and possess tapering ends. Fibres generally occur in groups, often located at the phloem poles of vascular bundles (Figures 2.2, 2.3, 2.6). The bulk of the secondary xylem (wood) in woody eudicots consists of fibres that confer considerable tensile strength. Bast fibres are formed in the secondary phloem and cortex in some species; they are used to make textiles or rope in species such as flax (Linum) and hemp (Cannabis). Gelatinous fibres are formed in the secondary xylem or sometimes in the bark of some woody plants; they are typically formed in response to stress caused by asymmetric branch thickening (compression wood or tension wood). In gelatinous fibres, the inner secondary wall is non-lignified and rich in polysaccharides, making it more flexibleReference Evert39.
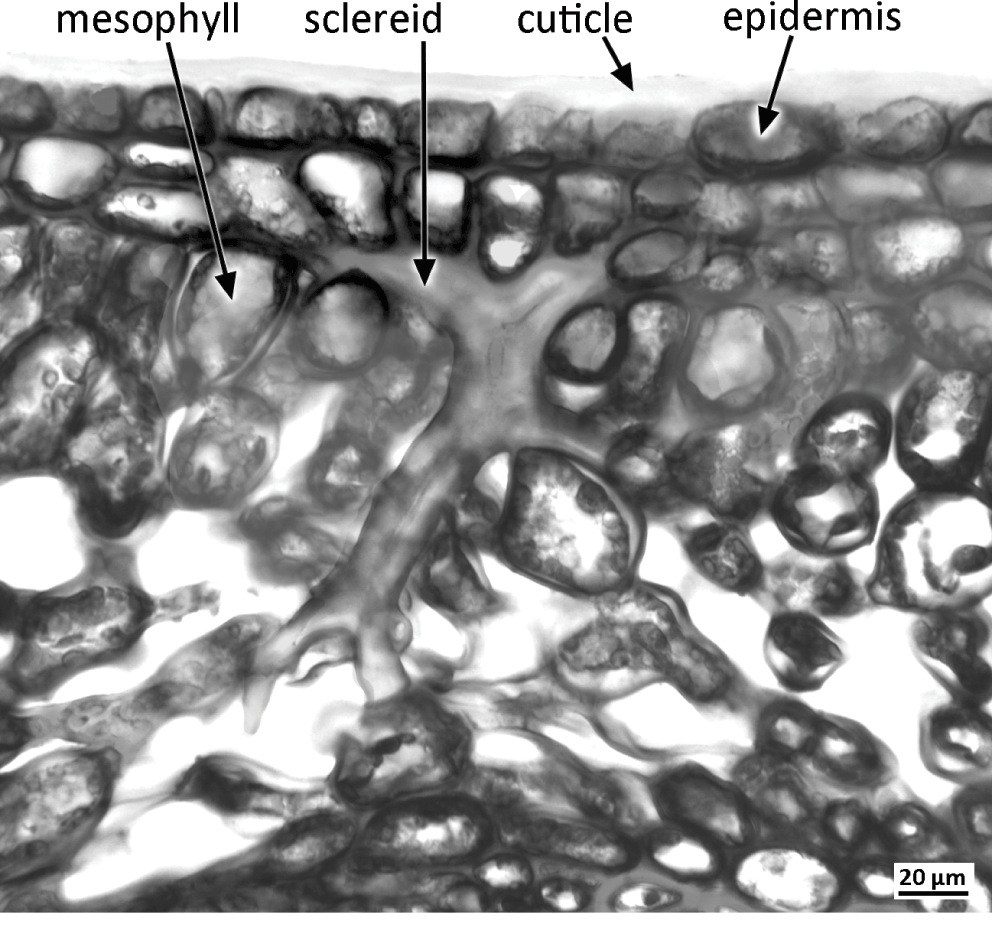
Sclereids (stone cells) are variously shaped cells that develop thick secondary walls as the plant matures; they can occur throughout the plant, either as isolated cells (idioblasts) or in groups (e.g. groups of stone cells in the fruit endocarp of peach). Brachysclereids are isolated isodiametric cells dispersed among parenchyma. Astrosclereids develop projections that grow intrusively into adjacent intercellular air spaces or along middle lamellae during the growth phase of the organ, prior to cell-wall thickening. The shapes of astrosclereids are dictated by the cellular arrangement of surrounding tissues; they are often star-shaped (Figure 1.9), though sclereids located in palisade mesophyll can be bone-shaped (osteosclereids).
1.8 Epidermis
The outermost (dermal) cell layer, the epidermis, covers the entire plant surface; in the root it is sometimes termed rhizodermis. The aerial epidermis is covered with a hydrophobic cuticle layer that consists primarily of a complex polymer, cutin, which forms a protective barrier to water and CO2 and also prevents adhesion between cells of adjacent organs in tightly packed structures such as the bud. The cuticle permeates the outermost cell wall and also forms an outer skin of varying thickness, often associated with epicuticular waxesReference Juniper and Jeffree70 (Figure 4.7). The cuticle can be striated or variously ornamented.
The epidermis is a primary tissue that is initially derived from the protodermal cells of the apical meristem. In developing organs, both anticlinal divisions and cell elongation extend the epidermis to accommodate organ elongation and even some organ thickening. The epidermis is usually uniseriate, with rare exceptions: a few species (e.g. Ficus elastica) develop a multiseriate epidermis in their leaves, and the aerial roots of many orchids possess a specialized multiseriate epidermis termed velamen. The epidermis generally persists throughout the life of the plant except in regions of lateral thickening growth, where it is often replaced by a periderm. In older roots, it is sometimes worn away by friction with the soil, and the root is subsequently protected by an exodermis formed by cell-wall thickening in the outer cortical layers.
The epidermis can develop various specialized cell types. For example, in leaves, relatively undifferentiated pavement epidermal cells are interspersed with several specialized cell types, including stomata and trichomes.
1.9 Stomata
Stomata are specialized pores in the epidermis through which gaseous exchange (water release and CO2 uptake) takes place. They occur not only on most aerial plant surfaces, especially on green photosynthetic stems and leaves, but also on some floral organs. Each stoma consists of two guard cells surrounding a central pore (Figures 1.10, 1.11). Guard cells are either kidney-shaped (in most plants) or dumbbell-shaped (in grasses, sedges and allied families); their inflation by increased osmotic potential results in opening of the pore, and their deflation causes its closure. Cuticular ridges extending over or under the pore from the outer or inner edges of the guard-cell walls help to seal the closed pore. In contrast with most epidermal cells, guard cells typically contain plastids that function as both chloroplasts and amyloplasts; these specialized plastids are smaller than those of mesophyll cells and contain photoreceptors that help to control stomatal openingReference Lawson80, Reference Sack, Zeiger, Farquhar and Cowan122.
Epidermal cells adjacent to the guard cells are termed subsidiary cells if their shape differs from that of other pavement epidermal cells; they can be either lateral or polar with respect to the guard cells. Specialized lateral subsidiary cells (e.g. in grasses and palms) can supply the guard cells with solutes that help to drive diurnal stomatal opening and closureReference Müller, Schäfer, Bauer, Geiger, Lautner, Fromm, Riederer, Bueno, Nussbaumer, Mayer, Alquraishi, Alfarhan, Neher, Al‐Rasheid, Ache and Hedrich90.
Classifications of stomatal types are based either on the presence and arrangement of mature subsidiary cells or on their patterns of developmentReference Payne99, Reference Rudall, Hilton and Bateman116, Reference Wilkinson, Metcalfe and Chalk156. Types of mature stomata include anomocytic (lacking subsidiary cells) and paracytic (possessing one or more pairs of lateral subsidiary cells oriented parallel with the guard cells). Lateral subsidiary cells of different species – though apparently similar – can have different developmental origins; mesogene lateral subsidiary cells are derived from the same cell lineage as the guard-mother cell, whereas perigene lateral subsidiary cells are derived from an adjacent cell lineageReference Payne99, Reference Rudall, Hilton and Bateman116. During development of the leaf epidermis in many eudicots (e.g. Arabidopsis), an iterative series of asymmetric divisions (amplifying divisions) results in a spiral arrangement of mesogene epidermal cells with a stomatal pore at the centre; the entire complex of cells is derived from the same initial cell and hence is monoclonalReference Nadeau and Sack92, Reference Rudall, Julier and Kidner117.
1.10 Trichomes and papillae
Epidermal outgrowths include papillae, trichomes (hairs) and specialized domed cells that characterize many petal surfaces. Papillae are small unicellular outgrowths from a single epidermal cell; some leaf surfaces possess multiple papillae per cell. Trichomes can occur on all parts of the plant surface; they vary widely in both form and function, partly depending on their location in the plant. Trichomes (Figure 1.12) can be unicellular or multicellular, branched or unbranched; specialized forms include root hairs (Chapter 3), glandular (secretory) hairs, scales, sensory hairs, stinging hairs (Figure 1.13) and hooked hairs that facilitate seed dispersal.

Figure 1.12 Plant hairs. (left) Quercus ilex (eudicot: Fagaceae), stellate non-glandular trichome on abaxial leaf surface. Scale = 50 µm. (right) Salvia involucrata (eudicot: Lamiaceae), trichomes on petal surface, including glandular (secretory) hairs with short stalks and either four-celled or single-celled glands. Scale = 20 µm.
Glandular trichomes usually possess a unicellular or multicellular stalk and a secretory head consisting of one or more cells. Secreted substances such as volatile oils collect between the secretory cells and a raised cuticle, which ultimately breaks to release the exudate. There are many different types of glandular hair that secrete a variety of substances, including salt, mucilage, essential oils and even enzymesReference Fahn41, Reference Werker154. Leaf glandular hairs of Cannabis sativa secrete a resinous substance containing cannabinoids that act as plant defense compounds. Glandular hairs of carnivorous plants such as Drosera secrete both sticky mucilage and proteolytic enzymes that help to capture and digest the prey. Salt-secreting glands help to modulate ion concentration in leaves of salt-tolerant plants such as the mangrove AvicenniaReference Juniper and Jeffree70.
Some trichomes are specialized for water absorption rather than secretion. Examples include the leaf scales that occur in many epiphytic and rock-dwelling Bromeliaceae, which represent an important source of water and mineral uptake to the plant. The specialized hairs (hydropotes) that occur on the submerged surfaces of many water plants (e.g. waterlily, Nymphaea) initially secrete mucilage, but following degeneration of the cap cells they absorb water and minerals and accumulate heavy metals.
1.11 Vascular tissue
Vascular tissue consists of associated networks of cells that conduct water (xylem) and nutrients (phloem). Primary vascular tissue is derived from the procambium, which is itself produced by the apical meristems. Secondary vascular tissue is derived from the vascular cambium in eudicots, and from the secondary thickening meristem in a few monocot species (Chapter 2.8). Both xylem and phloem are complex tissues composed of many different cell types.
1.11.1 Xylem
The primary function of xylem is transport of water throughout the plant, from roots via the stems to the leaves, where water is combined with carbon dioxide to make carbohydrates and oxygen during photosynthesis. Xylem is composed of several distinct cell types, often including parenchyma and fibres as well as vessels. Tracheids and vessel elements (collectively termed tracheary elements) are the water-conducting cells; they lack contents at maturity and are linked into cell chains to form vessels. Tracheary elements are elongated cells with thickened lignified walls. In a stem vascular bundle (Figure 1.14), the first-formed (protoxylem) elements often possess wall thickenings that are either helical or arranged in rings (annular). Later-formed primary tracheary elements (metaxylem) and secondary tracheary elements possess bordered pits in their lateral walls. Bordered pitsReference Choat, Cobb and Jansen18 can be oval, polygonal or elongated (scalariform); they can be organized in transverse rows (opposite pitting) or tightly packed (alternate pitting).

Figure 1.14 Vascular bundle. Lilium tigrinum (monocot: Liliaceae), transverse section of stem vascular bundle with xylem (left) and phloem (right) encircled by a ring of bundle sheath cells. bs = bundle sheath, c = companion cell, mx = metaxylem vessel, px = protoxylem vessel, s = sieve tube element. Scale = 50 µm.
The primary difference between tracheids and vessel elements is that vessel elements possess large perforations in their adjoining end walls, whereas perforations are absent from tracheids. Perforation plates are generally either simple, with a single opening, or scalariform, with a ladder-like row of openings divided by a series of parallel bars, or rarely a reticulate mesh. Vessel elements differ considerably in diameter, not only between different species but also sometimes across a single growth ring (e.g. in secondary xylem of oak).
1.11.2 Phloem
Phloem has complex roles in translocation of nutrients (sucrose and electrolytes) and hormones throughout the plantReference Evert39. Although commonly associated with xylem, phloem can develop precociously in regions that require a plentiful supply of nutrients, such as developing sporogenous tissue. Phloem consists of conducting cells (sieve elements) and associated specialized parenchyma cells (companion cells) (Figure 1.14); these two closely interdependent cell types are produced from a common parent cell (meristemoid) that divides and develops asymmetrically to form a larger sieve element and smaller companion cell. Most angiosperms possess sieve-tube elements rather than the relatively unspecialized sieve cells.
At maturity, sieve elements lack nuclei and most organelles but retain plastids and phloem-specific proteins (P-proteins). Companion cells are densely cytoplasmic, retaining nuclei and many active mitochondria. Sieve element plastids and P-proteins occur in several morphological forms (amorphous, filamentous, tubular and crystalline) that are often highly characteristic for particular plant families and are thus of systematic and evolutionary valueReference Behnke11, Reference Van Bel, Ehlers and Knoblauch145. Sieve-element plastids are classified according to their inclusions: starch (S-type plastids), protein (P-type plastids), or both.
Sieve elements are linked axially to form sieve tubes via sieve plates. Slime plugs are formed when P-protein accumulates on a sieve plateReference Evert39. The walls of sieve elements are thin and possess characteristic regions (sieve areas) that connect adjacent sieve elements; sieve areas consist of groups of pores and associated callose. In sieve tube elements, the sieve areas are localized on the adjoining end walls, forming sieve plates that are either simple or compound.
1.12 Secretory cells and laticifers
Secretory cells are specialized cells that release a broad range of substances, often targeted at a particular function. Secretory cells can occur throughout the plant body, either in the epidermis or in internal tissues. Epidermal secretory cells include glandular trichomes and nectaries, both extrafloral and floral. Internal secretory structures include idioblasts and specialized ducts or canals that are lined with cells that secrete substances such as resin or mucilage. Characteristic resin canals occur in the secondary xylem of some woody eudicots.
Laticifers are specialized latex-producing cells that permeate the tissues of a few angiosperms (Figure 1.7). Latex is an aqueous, often milky, solution that represents the source of commercial rubber, which is traditionally harvested from the rubber tree, Hevea brasiliensis. Laticifers of the opium poppy (Papaver somniferum) produce latex that is the source of narcotic analgesics such as morphine. Laticifers are traditionally classified into two cell types: non-articulated and articulated, though both types can occur within the same plant family (e.g. in Euphorbiaceae) and overlap in some of their propertiesReference Mahlberg86, Reference Rudall108, Reference Rudall110. Non-articulated laticifers (e.g. in Euphorbia, Figure 1.7) are highly branched cells that are derived from a small group of initial cells in the cotyledonary node of the embryo. These cells are coenocytes that undergo repeated nuclear divisions without corresponding wall formation. The resulting multinucleate cells grow intrusively between cells of surrounding tissues, branching frequently and eventually ramifying throughout the entire plant. In contrast, articulated laticifers (e.g. in Hevea brasiliensis) form complex syncytia by cell fusion and consist of linked chains of cells. Articulated laticifers can also grow intrusively between adjacent cells, though not to the same extent as the non-articulated cell type.