Highlights
Multisystem inflammatory syndrome in children and isolated viral myocarditis/myopericarditis could cause disturbances of the autonomic activity in the pediatric population, in which multisystem inflammatory syndrome in children is most prominent.
Heart rate variability is a non-invasive method to measure this dysfunction.
Impaired heart rate variability analysis could help clinicians in determining the risk of arrhythmia and, if necessary, initiating medical treatment by being followed in the ICU.
Myocardial involvement, whether or not is due to systemic inflammatory diseases or after isolated viral myocarditis/myopericarditis, can become lethal in some cases. Clues that can predict the clinical course seem to be very important in guiding the treatment of paediatric patients.
A novel new coronavirus came about in late 2019. It was called COVID-19, meaning coronavirus 2019, by The World Health Organization. The virus was officially designated as “severe acute respiratory syndrome coronavirus 2”. 1 The acute illness was usually mild in children, something that could not be set for their adult counterparts. After the acute phase, there could be a systemic inflammatory response with a severe shock-like illness in children with features of incomplete Kawasaki disease or toxic shock syndrome in seldom cases. Reference Riphagen, Gomez, Gonzalez-Martinez, Wilkinson and Theocharis2 This manifestation is called multisystem inflammatory syndrome in children.
This syndrome could be mild to severe in distinctive cases. It had the potential to involve all systems, but the cardiovascular system was its most regular target. Associated myocarditis and arrhythmia could cause lethal complications.
Isolated viral myocarditis/myopericarditis apart from multisystem inflammatory syndrome in children can also cause devastating events, but the prognosis depends on geography and/or the healthcare system available in each country. Reference Patel, Kelleman and West3,Reference Jain, Nolan and Singh4
Autonomic system response to systemic diseases could affect the course of the disease through the interaction of the respiratory, cardiovascular, and neurologic systems. Reference Scheer, Chellappa, Hu and Shea5 The release of various disease-related cytokines stimulates the sympathetic and parasympathetic systems. Interaction of the cardiovascular system and other organs with these systems can lead to an increased heart rate, arrhythmias, and other respiratory and central nervous system problems.
A non-invasive biomarker, heart rate variability, could be a good indicator of the function of the autonomic system and could also help us to more accurately estimate cardiac and other system complications. Reference Leitzke, Stefanovic, Meyer, Schimpf and Schönknecht6 Heart rate variability is related to modifications in the relaxation and stress status of individuals within the sympathetic and parasympathetic autonomic nervous systems.
Analysis of heart rate variability parameters will also contribute to the differentiation of the diseases that are similar to one another. These parameters may help interpret clinical courses of other diseases with myocarditis involvement, such as Kawasaki disease or acute rheumatic fever.
This study aimed to analyse the autonomic function by heart rate variability, correlate it with other laboratory and echocardiographic findings, and compare these results among multisystem inflammatory syndrome in children, isolated viral myocarditis/myopericarditis, and control groups to predict the course and customise the differential diagnosis and treatment of the diseases with myocarditis/ myopericarditis.
Methods
Patients diagnosed with multisystem inflammatory syndrome in children, isolated viral myocarditis/myopericarditis, at a university hospital from September 2021 to February 2023 were included in this study. The control group, who were compatible in age and gender with the patient groups, were selected from healthy subjects that applied to the hospital for murmur, chest pain, or palpitation. The local ethics committee approved the protocol. The Helsinki Declaration was taken into consideration. Written informed consent was obtained from the parents of all participants.
Multisystem inflammatory syndrome in children is diagnosed according to the Centers for Disease Control and Prevention and the World Health Organization criteria. 7,8 Both clarifications include fever (though they alter concerning duration), increased inflammatory markers, at least two signs of multisystem involvement, clue of severe acute respiratory syndrome coronavirus 2 infection or exposure, and exclusion of other potential sources. Multisystem inflammatory syndrome in children patients were subgrouped according to the established criteria as mild or severe form. Reference Brisca, Consolaro and Caorsi9 Some patients with a more serious clinical course were evaluated in the severe form according to the predominance and severity of cardiac functions, cardiac enzyme levels, brain natriuretic peptide (BNP) values, accompanying hypotension, arrhythmias, and non-cardiovascular gastrointestinal system involvement. In another classification, multisystem inflammatory syndrome in children is divided into groups according to its overlapping with Kawasaki disease and acute COVID-19 infection. Reference Godfred-Cato, Bryant and Leung10 Inclusion criteria were to be diagnosed and followed up in this single-centre clinic.
Myocarditis/myopericarditis diagnosis is made according to the algorithm of the American Heart Association and European Society of Cardiology. Reference Law, Lal and Chen11,Reference Adler, Charron and Imazio12 It includes history, clinical, and laboratory findings with the addition of cardiac magnetic resonance imaging confirming the diagnosis. Reference Butts, Boyle and Deshpande13,Reference Caforio, Pankuweit and Arbustini14 All patients had chest pain and elevation of cardiac biomarkers (troponin I and creatinin kinase-myocardial band). When there is associated pericarditis, it is called myopericarditis. There was a viral prodrome within 1–4 weeks in all patients. Electrocardiography showed ST segment changes/ T wave inversion.
The inclusion criteria for the control group were that they could have no other cardiologic diseases including structural heart diseases and arrhythmia syndromes.
Complete blood count, acute phase reactants, creatinine kinase, cardiac biomarkers (troponin I and creatinin kinase-myocardial band), and N terminal-pro-Brain natriuretic peptide were also evaluated in children alongside multisystem inflammatory syndrome in children and myocarditis/myopericarditis.
The exclusion criteria for patients and the control group were that they have other systemic diseases. Children with chronic illnesses and those taking daily medications were, therefore, excluded from the study.
Electrocardiography (Standard 12-lead electrocardiography) was performed for the patients at a paper speed of 25 mm/second under similar conditions. A Nihon Kohden ECG 1250 Cardio fax S (2009, Tokyo, Japan) device was used at standard velocity and amplitude, with 24-hour rhythm Holter analysis (Century Holter model 3000 system). Transthoracic echocardiography, performed via Vivid E9 Pro Ultrasound System (GE Medical Systems, Canada) by using 3 and 6 MHz transducers as 2D, M-mode and coloured Doppler, conventional continuous-wave and pulse wave Doppler visualising methods. Two experienced paediatric cardiologists performed all studies.
Recordings taken with a vx3+ model Century Holter model 3000 system solid-state recorder were evaluated via computer using the same software system. Twenty-four-hour Holter monitorization was performed on the median 3rd day ( 2–7 days) of the disease after the patients were given acute treatment. Blood pressure readings of all patients were in the normal range, and none of them had been receiving inotropes or other cardiovascular medications during Holter monitoring.
Heart rate variability analysis
Heart rate variability is a plain, non-invasive, objective, and validated measuring procedure for the evaluation of the autonomic nervous system function. 15,Reference Asarcikli, Hayiroglu, Osken, Keskin, Kolak and Aksu16
Heart rate variability is acquired from intervals between normal sinus heartbeats and can be quantified using a variety of methods [6,20,38-43], primarily comprising: time-domain measures, frequency-domain measures, and heart rate turbulence. Time-domain and frequency-domain methods are based on the measurement of changes in consecutive RR intervals on 24-hour rhythm Holter recordings.
Time-domain study
Analyses were conducted on the variation of the heart rate during a standard time interval based on the time elapsed between two successive R-waves of the QRS signal on the electrocardiogram, R wave to R wave (RR) distances between two consecutive sinus beats. These parameters included the standard deviation of normal to normal intervals (SDNN), the standard deviation of 5-minute RR interval means (SDANN), the mean of the 5-minute RR interval standard deviations (SDNNi), root mean square of successive RR interval differences (rMSSD) and the percentage of the beats with consecutive RR interval difference of more than 50 ms (pNN50).
Frequency-domain study
This was analysed by periodic signals, an average of 500 sequential RR intervals divided into various bands of frequency response. Total power (TP, the area under the spectral curve from 0.01 to 1.0 Hz), very low-frequency power (the area under the spectral curve from 0.0033 to 0.04 Hz, very low frequency), low-frequency (the area under the spectral curve from 0.04 to 0.15 Hz, low frequency), and high-frequency band power (the area under the spectral curve from 0.15 to 0.40 Hz, high frequency) were examined and the low frequency/high-frequency ratio was calculated.
Frequency-domain parameters include low-frequency and high-frequency bands in spectral analysis. Low-frequency bands are connected to the sympathetic nervous system, including the parasympathetic component. Very low-frequency bands are bonded to parasympathetic deactivation (17).
The high frequency reflects parasympathetic activity, whereas low frequency shows both sympathetic and parasympathetic activity, and SDNN, rMSSD, and pNN50 describe the parasympathetic activity. Reference Nunan, Sandercock and Brodie17 The triangular index is a basic marker for heart rate variability and is repressed by sympathetic impacts. The low frequency is the only parameter to calculate the activity of sympathetic activity. The low frequency/high-frequency index is between 0.15 and 0.4. Low frequency/high-frequency ratio is assessed to preclude parasympathetic constituents in the low frequency. Whilst high low frequency/high-frequency ratios show a rise in sympathetic activity, low ratios reflect a rise in parasympathetic events. It is related to the modulation of the efficacy of gas exchange, respiratory sinus arrhythmia, parasympathetic nervous system activity, and innervation of the vagus nerve. Premature ventricular beats are eliminated in the heart rate variability analysis. Reference Lippman, Stein and Lerman18
Statistical analysis
Categorical data were summarised through numbers and percentages. Normal distribution control of continuous data was done with Shapiro-Wilk’s test. Continuous data, that are not suitable for normal distribution according to mean and standard deviation data, were summarised using median and quartiles. Group comparisons were made with analysis of variance or the Kruskal-Wallis test.
In addition, the relationships between heart rate variability parameters and laboratory, echocardiographic measurements were evaluated with Spearman correlation coefficients. Statistically significant correlations greater than 0.40 were interpreted. Diagnostic performances of heart rate variability and electrocardiography parameters were evaluated using Receiver Operating Curve analysis. The sensitivity and specificity values for the obtained cut-off values were summarised. The statistical significance value was taken as p < 0.05. Statistical analyses were performed with the STATISTICA 13.0 package programme. Power analysis is done to determine the number of patients and control participants.
Results
There were 30 multisystem inflammatory syndrome in children patients, 43 patients with myocarditis, and 109 patients in the control group. Eleven patients had a severe type of multisystem inflammatory syndrome in children, of whom 9/11 patients had severe Kawasaki-like type multisystem inflammatory syndrome in children, and 2 patients had hyperinflammatory type without overlap with Kawasaki disease or acute COVID-19.
The mean age was 139 ± 49.1 months (5–17 years old) in multisystem inflammatory syndrome in children, 141.8 ± 61.59 months (7–18 years old) in myocarditis, and 137.47 ± 58.02 months (5–17 years old) in the control group.
Imaging and other examination results
The echocardiographic evaluation of all control participants was normal. Left ventricular systolic function slightly decreased (ejection fraction 47–55%), in 5 patients with multisystem inflammatory syndrome in children; while it was in 3 patients with myocarditis/myopericarditis.
The median ventricular ejection fraction was significantly lower in the multisystem inflammatory syndrome in children group (66.5% versus 71%; P = 0.018) and the median and z score of left ventricular end-diastolic dimension was higher in the isolated myocarditis/myopericarditis group compared to multisystem inflammatory syndrome in children (median left ventricular end-diastolic dimension: 42 mm–39 mm z score: +0.1 versus −0.12; P = 0.035), respectively.
Although systolic and diastolic blood pressures were lower in multisystem inflammatory syndrome in children patients than in the others, the range was normal in all three groups. The baseline characteristics of all participants are summarised in Table 1.
Table 1. Demographic features and physical examination results of the groups

Cardiac MR was performed in 30 of the patients with myocarditis and 14 of the patients with multisystem inflammatory syndrome in children. Because of the difficulty of the imaging technique for children, the procedure could not be completed in the remaining patients. However, these patients who did not undergo MR imagination were diagnosed by the guidelines using other history, clinical and laboratory findings. Late enhancement positive or T1 mapping positive myocarditis findings were observed in all patients who underwent cardiac MRI, and myocardial involvement was observed in these patients.
24-hour Holter monitoring and heart rate variability
During the 24-hour rhythm Holter monitoring evaluation, 11 patients had rare extra ventricular beats and two patients had non-sustained 8–9 beats ventricular tachycardia in the multisystem inflammatory syndrome in children study group, and nine patients had rare ventricular extrasystole and one patient had non-sustained ventricular tachycardia 5–6 beats in a row in myocarditis/myopericarditis group. In the control group, 35 participants, 13 with rare extra ventricular beats and the other with rare extra supraventricular beats, were found. Heart rate variability parameters including both time-domain and frequency-domain components are summarised in Table 2.
Table 2. Heart rate variability parameters including both time–domain and frequency–domain components and physical examination measurements and comparison among MIS–C, myocarditis, and control groups

bpm = beat per minute; ms = miliseconds.
Statistically significant differences were found in SDNN, SDNNİ, SDANN, RMSSD, pNN50%, very low frequency, high frequency, low frequency, and triangular index in multisystem inflammatory syndrome in children patients, who had impaired and declined heart rate variability values compared to the other two groups. Low frequency/high-frequency ratio was meaningfully higher in patients with multisystem inflammatory syndrome in children. In patients with myocarditis, although SDNN, SDNNI, SDANN, RMSSD, and triangular index were also lower, no significant difference was detected in these parameters within the control group.
When the mild and severe forms of multisystem inflammatory syndrome in children were compared with each other for the HRV parameters, the only significant difference appeared in triangular index (p < 0.05). While the median of triangular index was 29.5 in the mild form, it was 10 in the severe form. The discriminating power of heart rate variability parameters on patients with multisystem inflammatory syndrome in children was evaluated. The predictivity of the SDNN, SDNNI, SDANN, RMSSD, pNN50%, very low frequency, high frequency, low frequency, low frequency/high frequency, and triangular index in heart rate variability was higher according to others. According to this model, cut-off values were calculated. Individuals with values below these heart rate variability cut-off parameters were classified as multisystem inflammatory syndrome in children. All cut-offs and p-values are explained in Table 3.
Table 3. Cut-off parameters of HRV values for classifying as MIS-C.
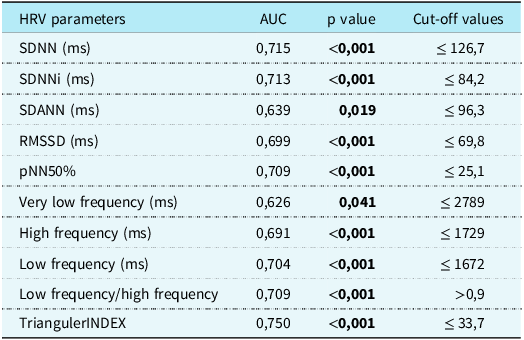
AUC = area under curve; HRV = heart rate variability; MIS-C = multisystem inflammatory syndrome in children.
Neither cardiac MRI ejection fraction nor the existence of arrhythmia made a significant difference in the heart rate variability parameters.
Laboratory biomarkers
Troponin I, creatinin kinase, and creatinin kinase-myocardial band levels were 76 higher in both myocarditis and multisystem inflammatory syndrome in children groups and N terminal-pro-Brain natriuretic peptide was high in twenty-three patients of multisystem inflammatory syndrome in children, and fifteen patients of myocarditis.
When we look at the relationship between laboratory values and heart rate variability, no significant correlation was found between cardiac biomarkers and N terminal-pro-Brain natriuretic peptide in both multisystem inflammatory disease and myocarditis.
In the myocarditis group, there was a positive correlation between white blood cells and SDNN, SDNI, RMSSD, pNN50, high frequency, and low frequency (r = 0.9989–1; p < 0.05).
Other correlation results
In the MISC group, there was a negative correlation between pNN50% and left ventricular end-diastolic dimension (r = −0.600, p = 0.000), and a positive moderate correlation with ejection fraction and fractional shortening (r = 0.439, and r = −0.400; P = 0.015 and P = 0.028, respectively). There was an intermediate negative correlation between left ventricular end-diastolic dimension and maximum HR in multisystem inflammatory syndrome in children and a weak negative correlation in myocarditis due to the control group (r = −0.306, and r = −0.471, respectively; P = 0.009, P = 0.046). Patients are followed for a median of 1 year (6 months-2 years). There were neither fatal arrhythmia nor ventricular systolic dysfunction in the long-term results.
Discussion
In this study, the impairment in heart rate variability parameters was significant in multisystem inflammatory syndrome in children patients compared to myocarditis and the control group. In patients with myocarditis, even if the heart rate variability parameters also diminish that show autonomic system dysfunction, the difference wasn’t prominent. Considering the correlation between heart functions and heart rate variability values, heart rate variability parameters improve as heart contraction improves and left ventricular diameter decreases.
All these factors represent an important risk increase in cardiac-related complications like arrhythmia, and sudden cardiac death in patients with multisystem inflammatory syndrome in children. In addition, this autonomic dysfunction could also cause extra co-morbidities involving, especially neurologic, respirator, and other systems. There have been some previous studies comparing myocarditis and multisystem inflammatory syndrome in children in terms of clinical signs and prognosis. After reviewing the literature, it appears this is the first study assessing these values in multisystem inflammatory syndrome in children and comparing them with isolated viral myocarditis.
Multisystem inflammatory disease in children reflects a wide clinical spectrum that involves, most importantly, the cardiovascular system. Cardiovascular system complications do not affect all patients in the same manner. Reference Alsaied, Tremoulet and Burns19 Myocarditis is also an inflammatory process localised to the heart muscle. A prognostic and predictive value would be beneficial to clinicians in follow-up and the prescription of medication. Dysfunction of the autonomic nervous system could lead to abnormalities in sympathovagal balance. This interaction might cause respiratory, cardiovascular, and neurologic system problems. Reference Stein20 It could precipitate a distinctive course in diseases. This study pointed to the difference in autonomic nervous system response between these two patient groups.
Normal ranges for heart rate variability in children are uncertain but can be determined by both comparing adult studies and data from other healthy children. In the rare paediatric studies that do exist, no significant relationship was found between age, gender, height, and weight parameters, and reference to heart rate variability values. Reference Seppälä, Laitinen and Tarvainen21,Reference Gąsior, Sacha and Pawłowski22 Despite these findings, another study suggested that the heart rate variability parameters were age and sex dependent. Reference Silvetti, Drago and Ragonese23 Age and sex similarity between groups were taken into account in the present study and there was no significant difference between the groups. A reassessment of other studies of normal values for short-term heart rate variability found huge differences in values. Reference Nunan, Sandercock and Brodie17 It seems better to use longer records such as 24-hour Holter monitoring to evaluate heart rate variability like the way in this work. Prognostically, reductions in heart rate variability are independent predictors of overall mortality, mortality from heart failure, sudden cardiac death, ventricular arrhythmias, and the need for transplant. Reference Fauchier, Babuty, Cosnay and Fauchier24 Both domains of heart rate variability parameters: the time and frequency domains are used in this study, as it is better to have different aspects of cardiac autonomic activity, that could give us better predictive value. When the SDNN value is over 100 ms, it has been associated with a markedly lower risk of mortality. Reference Nolan, Batin and Andrews25 In our study, we use a healthy control group to evaluate heart rate variability parameters. A decrease in heart rate variability parameters compared to healthy patients was considered significant evidence for corruption in heart rate variability.
Time-domain and frequency-domain features of heart rate variability like SDNN, SDNNİ, SDANN, RMSDD, pNN50%, very low frequency, and high frequency that show parasympathetic activity diminished prominently in patients with multisystem inflammatory syndrome in children. Even if low frequency shows both sympathetic and parasympathetic activity, an increase in the ratio of low frequency/high frequency demonstrates a sympathetic activity rise in these patients. All these factors could lead to multisystemic dysfunction including cardiac, neurologic, and gastrointestinal systems that are in contact with the autonomic nervous system. Severe form of multisystem inflammatory syndrome in children had more impaired and declined heart rate variability parameters, especially in the triangular index. Although a significant difference wasn’t obvious in all parameters, it could be interpreted that as the severity of the disease increases, the autonomic dysfunction could get more impaired. The results could be more meaningful with a slightly larger group.
In a study comparing myocarditis and multisystem inflammatory syndrome in children, it was reported that the clinical course and later recovery were better in multisystem inflammatory syndrome in children patients. Reference Patel, Kelleman and West26 Myocarditis could lead to a worse course including left ventricular systolic dysfunction. In our study, heart rate variability parameters were more impaired in multisystem inflammatory syndrome in children patients than in myocarditis and control groups. Although there were effects on heart rate variability values due to the deterioration of the autonomic nervous system in both disease groups, this deterioration was significant in multisystem inflammatory syndrome in children patients.
There was also a decrease in heart rate variability values in myocarditis, but no significant difference was found when compared to healthy patients. In previous studies, it was observed that there was an impairment in heart rate variability of myocarditis, especially in cases with arrhythmia. Reference Ling, Li, Wang, Zhang, Xu and An27 It can be said that heart rate variability parameters are prone to severe arrhythmia and heart failure. It should be kept in mind that multisystem inflammatory syndrome in children patients with myocardial involvement may be more prone to the risk of cardiac arrhythmia and sudden cardiac events.
As multisystem inflammatory syndrome in children is a systemic illness with multiple factors at play, it could lead to severe left ventricular systolic dysfunction and also arrhythmia. Comparing myocarditis associated with other systemic involvements, which are different from isolated viral myocarditis/myopericarditis, may also be a guide in terms of treatment plans. The excess of cardiac involvement in these patients may also be related to dysfunction of the autonomic nervous system. Close follow-up in the ICU could be preferable, and 24-hour Holter monitoring and anti-arrhythmic drugs should be considered in patients with increased cardiovascular risk.
Looking at the correlations, considering the laboratory parameters, no significant relationship was found between the expected cardiac markers and BNP values. There could be leukopoenia in isolated viral myocarditis, and the positive correlation between white blood cells and heart rate variability in these patients could be explained by this association. We noted that heart rate variability parameters improved as cardiac functions improved. We found left ventricular end-diastolic dimension and ejection fraction were correlated to impaired maximum heart rate and heart rate variability parameters, similar to the findings of other studies. Reference Kleiger, Miller, Bigger and Moss28 As the functions of the heart muscle decrease and the diameter of the left ventricle increases, more attention should be paid to cardiac effects and arrhythmias in these patients by looking at the electrocardiography parameters. Anti-arrhythmic treatments, such as beta-blockers, can be started for prevention in these patients whose cardiac functions are affected after 24-hour Holter monitoring and electrocardiography evaluations.
Other studies also investigate if heart rate variability measurements on any disease could predict any cardiotoxic involvement. Reference Sullivan, Grice, Lake, Moorman and Fairchild29,Reference Mol, Strous and van Osch30 A significant decrease in heart rate variability parameters can play a role in cardiac arrhythmias and predict deterioration in cardiac functions, especially in multisystem inflammatory syndrome in children patients. These heart rate variability parameters are thought to be useful in the differential diagnosis of myocardial involvement in systemic diseases such as multisystem inflammatory syndrome in children, apart from isolated viral myocarditis/myopericarditis.
Finally, further large sample works should be accompanied to analyse the clinical usage of heart rate variability in different clinical situations and also other neuroautonomic impacts.
Limitations
This is a single-center study and these diseases are so scarce, especially multisystem inflammatory syndrome in children. Other limitations arise from the method of the 24-hour rhythm Holter monitoring approach, which can be altered by all physical events and the posture of subjects and sleep actions.
In conclusion, both multisystem inflammatory syndrome in children and isolated viral myocarditis could cause disturbances of the autonomic activity in the paediatric population, in which multisystem inflammatory syndrome in children is most prominent. Patients with impaired left ventricular dysfunction and enlargement in the left ventricular dimension could be screened by 24-hour Holter monitoring. These values will be useful in determining the risk of arrhythmia, cardiac, and other systemic effects. Randomised controlled studies with a larger number of patients should be done in this manner.
There is no conflict of interest and no financial disclosure.
Data availability
We assure the Editorial Board that the results presented in this paper have not been published previously in whole or part, except in abstract form, and are not currently under consideration for publication elsewhere.
Author contribution
Derya Duman drafted the manuscript; Derya Duman and Derya Karpuz performed the procedure and collected the data; Bahar Taşdelen made the statistical design of the study; Necdet Kuyucu, Derya Karpuz, Bahar Taşdelen, and Derya Duman designed this research and revised the manuscript; all authors read and approved the final version of the manuscript.
Financial support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare no conflicts of interest concerning the research, authorship, and/or publication of this article.
Ethical approval
The local ethics committee approved the protocol (Decision date and number: 8/9/2021-608). The Helsinki Declaration was taken into consideration. Written informed consent was obtained from the parents of all participants.






