Anaemia is prevalent in seriously ill children, with a reported incidence of 75% in patients staying in the paediatric intensive care unit for more than 2 days, and it is associated with increased mortality ranging from 20 to 50%. Reference Bateman, Lacroix and Boven1,Reference Carson, Noveck and Berlin2 In paediatric critical care, unnecessary blood transfusions significantly predict morbidity and mortality. While transfusion-related lung injury is a well-known complication in patients requiring transfusion, transfusion-associated circulatory overload is more commonly observed in patients receiving transfusions during their critical care follow up. Reference Lacroix, Hébert and Hutchison3 Transfusion is a life-saving intervention in cases of haemorrhagic shock and serious anaemia (haemoglobin level < 5.0 g/dL). However, the question remains: to what extent should anaemia be tolerated in haemodynamically stable, non-bleeding, critically ill children?
Two different transfusion approaches have been suggested in studies: liberal transfusion (haemoglobin < 9.5 g/dL) and restrictive transfusion (haemoglobin < 7.0 g/dL). Reference Klaus, Frank and Salazar4 Cholette et al. demonstrated that restrictive transfusion had a similar effect to liberal transfusion in terms of rates of progressive multiple organ failure, with a 50% reduction in the incidence of red blood cell transfusions by setting the haemoglobin threshold for transfusion at 7.0 g/dL. Reference Cholette, Powers and Alfieris5 While restrictive transfusion has gained acceptance as a strategy for haemodynamically stable critically ill paediatric patients, a consensus on the transfusion approach in patients with CHD was reached only recently. Reference Valentine, Bembea and Muszynski6 The literature has expanded since establishing the Paediatric Critical Care Transfusion and Anaemia Expertise Initiative in 2018, providing recommendations to clinicians. Reference Cholette, Willems and Valentine7 Nonetheless, few studies on the transfusion approach in the post-operative management of CHD remain. Therefore, this study aimed to compare liberal and restrictive transfusion practices in post-operative CHD patients in the cardiac intensive care unit.
Materials and method
Before 2019, our institution did not follow specific transfusion guidelines in the cardiac intensive care unit and the operating room. Between 2019 and 2022, blood products began to be utilised based on the recommendations of the Society of Cardiovascular Anesthesiologists Continuous Practice Improvement Blood Conservation Working Group, as published in 2019. Reference Raphael, Mazer and Subramani8 However, a consensus on red blood cell transfusion was not reached until 2021. Since 2021, a restrictive strategy has been implemented for red blood cell transfusion in the operating room, cardiac intensive care unit, and inpatient wards.
Regarding other blood products, the protocol specifies that fresh frozen plasma is used in cases of severe bleeding and when there is an increase in international normalised ratio and activated partial thromboplastin time, but it is not recommended as a volume expander. Platelet concentrate is administered to patients with a platelet count below 50,000 experiencing oozing or those with a platelet count below 100,000 experiencing severe bleeding or exhibiting a clinical picture suggestive of platelet dysfunction. Cryoprecipitate (Cryo) is not advised for prophylactic use but rather reserved for patients with a fibrinogen level below 150 mg/dL and post-cardiopulmonary bypass bleeding.
The definition of liberal transfusion is the administration of transfusion when the haemoglobin level is below 9.5 g/dL without considering clinical parameters (such as end-organ function and cardiac contractility), laboratory indicators (like lactate and mixed venous saturation), and haemodynamic factors (including blood pressure and heart rate). Conversely, restrictive transfusion sets the haemoglobin threshold at 7.0 g/dL for haemodynamically stable patients with sufficient oxygenation (normal lactate and mixed venous saturation) and normal end-organ function. This retrospective observational study analysed CHD patients who received a liberal transfusion from 2019 to 2021 and those who received a restrictive transfusion from 2021 to 2022. Haemodynamically unstable patients who had active bleeding or haemorrhagic shock and required urgent transfusion were given blood regardless of their haemoglobin level. The study included patients aged 1 month to 18 years undergoing CHD operations, excluding those younger than 1 month old and those with single ventricle physiology. Both groups were retrospectively compared and analysed based on patient characteristics (age, gender, weight, body mass index, CHD type [cyanotic versus acyanotic], and Risk Adjustment for Congenital Heart Surgery-1 score), reoperative cardiac surgery (redo), intraoperative variables (cardiopulmonary bypass time and aortic cross-clamp time), post-operative variables (Paediatric Risk of Mortality-3 score, Paediatric Logistic Organ Dysfunction-2 score, vasoactive inotropic score, total fluid balance, total duration of mechanical ventilation, duration of high flow oxygen therapy, length of cardiac intensive care unit stay), as well as pre-operative anaemia, pre-operative haemoglobin level, first post-operative haemoglobin level, minimum and maximum haemoglobin levels during cardiac intensive care unit stay, hospital discharge haemoglobin levels, first post-operative international normalised ratio, activated partial thromboplastin time and fibrinogen levels, number of transfused red blood cells, and other blood products (cryoprecipitate [Cryo], fresh frozen plasma, platelet concentrate).
Additionally, haemoglobin and lactate levels at the time of and after red blood cell transfusion were recorded. The study also analysed clinical features such as transfusion reactions, bleeding, re-operation, the need for dialysis, the need for extracorporeal membrane oxygenation, acute kidney injury, and organ-specific complications (neurological, haematological, coagulopathy, hepatic, sepsis, and systemic inflammatory response syndrome). Unfortunately, our hospital does not currently utilise cell saver and viscoelastic tests such as rotational thromboelastometry and thromboelastography. There were no changes in the number of surgeons, anaesthesiologists, or intraoperative techniques, including cardiopulmonary bypass priming (prime volume and ultrafiltration), between 2019–2021 and 2021–2022. The study received approval from the Koc University Ethics Committee on Human Research (Approval Number: 2022.259.IRB1.100).
Tools and definitions
The number of transfused blood products was determined as follows:
-
Each 10 mL/kg of red blood cell transfusion was counted as one red blood cell transfusion.
-
For platelet concentrate, each 10 mL/kg of platelet concentrate transfusion was counted as one platelet concentrate transfusion.
-
For fresh frozen plasma, each 10 mL/kg of fresh frozen plasma transfusion was counted as one fresh frozen plasma transfusion.
-
For cryoprecipitate (Cryo), each 10 mL/kg of Cryo transfusion was counted as one Cryo transfusion.
-
Anaemia is traditionally defined as a decrease in red blood cell volume or haemoglobin and hematocrit concentration more than two standard deviations below the mean for age and gender. Reference Gallagher9
-
Δ Hb means pre-operative haemoglobin – first post-operative haemoglobin.
-
Bleeding is defined as hourly chest drainage exceeding 3 mL/kg/h for three consecutive hours after surgery or marked drainage of 5 mL/kg/h in any 1 h without clotting. Reference Backer, Costello, Kane, Mavroudis, Mavroudis and Backer10
The Paediatric Risk of Mortality-3 score and the Paediatric Logistic Organ Dysfunction-2 score are scoring systems used to predict the risk of mortality based on the severity of organ dysfunction. Calculations were performed using the worst parameters within the first 24 hours of admission. Reference Pollack, Patel and Rutimann11,Reference Leteurtre, Duhamel and Salleron12
Single or multiple organ failure (neurologic, respiratory, gastrointestinal, hepatic, haematologic, and coagulopathy) was defined by using Pediatric Organ Dysfunction Information Update Mandate contemporary organ dysfunction criteria. Reference Bembea, Agus and Akcan-Arikan13
Acute kidney injury was assessed according to the Kidney Disease Improving Global Outcomes criteria, with acute kidney injury categorised into stages 1–3 based on changes in serum creatinine and/or urine output. 14
Patients with a mean arterial pressure below two standard deviations from the normal range for their age were considered hypotensive and started on inotropic agents. The inotropic score in these patients was calculated using the vasoactive inotropic score, with the maximum value (VISmax) included in the analysis. Reference Gaies, Gurney and Yen15
The diagnostic criteria from the “International Paediatric Sepsis Consensus Conference in 2005” were utilised for diagnosing sepsis and systemic inflammatory response syndrome. Reference Goldstein and Giroir16
Statistics
The data were analysed using Statistical Package for Social Sciences for Windows version 22.0 software. Descriptive statistics were used for data evaluation, including frequency, percentages, mean, median, and standard deviations. Before hypothesis testing, the distribution of numerical data and homogeneity of variance were assessed using the Kolmogorov-Smirnov and Levene’s tests, respectively. When both conditions were met, the independent samples t-test was employed to compare continuous data between two independent groups. Alternatively, the Mann-Whitney U test was utilised when these conditions were not met. Categorical data were analysed using the chi-square (χ2) test, and a significance level of p < 0.05 was considered statistically significant.
Results
Of the 96 included patients, 53 were analysed in the restrictive transfusion group, while 43 patients were analysed in the liberal transfusion group. As shown in Table 1, there were no differences in age, gender, body mass index, cyanotic patients, Risk Adjustment for Congenital Heart Surgery-1, redo, cardiopulmonary bypass time, aortic cross-clamp time, VISmax, Paediatric Risk of Mortality-3, Paediatric Logistic Organ Dysfunction-2, total fluid balance, duration of mechanical ventilation, high flow oxygen therapy duration, length of stay, and, as well as, mortality. The pre-operative haemoglobin level of the liberal group was higher than that of the restrictive group (13.80 ± 2.44 and 12.59 ± 2.60, p = 0.02). Comparing the perioperative use of blood products in cardiopulmonary bypass priming between the restrictive and liberal groups, it was found that 84.9% of patients in the restrictive group (n = 45) were primed with red blood cells, whereas this number was 67.4% in the liberal group (n = 29) (p = 0.043). Concerning the volume of red blood cells used in priming, the restrictive group, which received red blood cell priming, had a mean of 199.33 ± 130 mL (Median: 200 mL) of red blood cells, while the liberal group had 140.93 ± 126.9 mL (Median: 150 mL) (p = 0.030). When comparing the use of fresh frozen plasma in priming between the two groups, 34% of patients in the restrictive group (n = 18) received fresh frozen plasma, while it was 25.6% in the liberal group (n = 11) (p = 0.37). The volumes of fresh frozen plasma used in the restrictive and liberal groups were 168.88 ± 93.42 mL (Median: 175 mL) and 155.45 ± 90.92 mL (Median: 150 mL), respectively (p = 0.70). During cardiopulmonary bypass priming, only 18.9% of the restrictive group received albumin, while it was 9.3% in the liberal group (p = 0.18) (Table 1).
Table 1. Characteristic features of the patients in the study group.

BMI=body mass index; CICU=cardiac intensive care unit; CPB=cardiopulmonary bypass; FFP=fresh frozen plasma; HFOT=high flow oxygene theraphy; MV=mechanical ventilation; n=number of patients; PRISM-3=paediatric risk of mortality-3 score; PELOD-2 score=paediatric logistic organ dysfunction score-2; RACHS-1=risk adjustment of congenital heart surgery score; Redo=reoperative heart surgery; RBC=red blood cell; VIS=vasoactive inotropic score.
*p < 0.05 was statistically significant.
When comparing the first post-operative haemoglobin level, it was significantly higher in the liberal group (12.03 ± 1.62) compared to the restrictive group (10.39 ± 1.67, p < 0.001). The liberal group also showed higher haemoglobin levels for the minimum and maximum post-operative haemoglobin levels during their cardiac intensive care unit stay (p < 0.001 and p = 0.019, respectively). However, there were no differences in hospital discharge haemoglobin levels between the two groups (restrictive: 11.46 ± 1.49, liberal: 11.42 ± 1.24, respectively, p = 0.86) (Table 2).
Table 2. Pre-operative and post-operative haemoglobin levels and laboratory parameters of the patients in the study group.
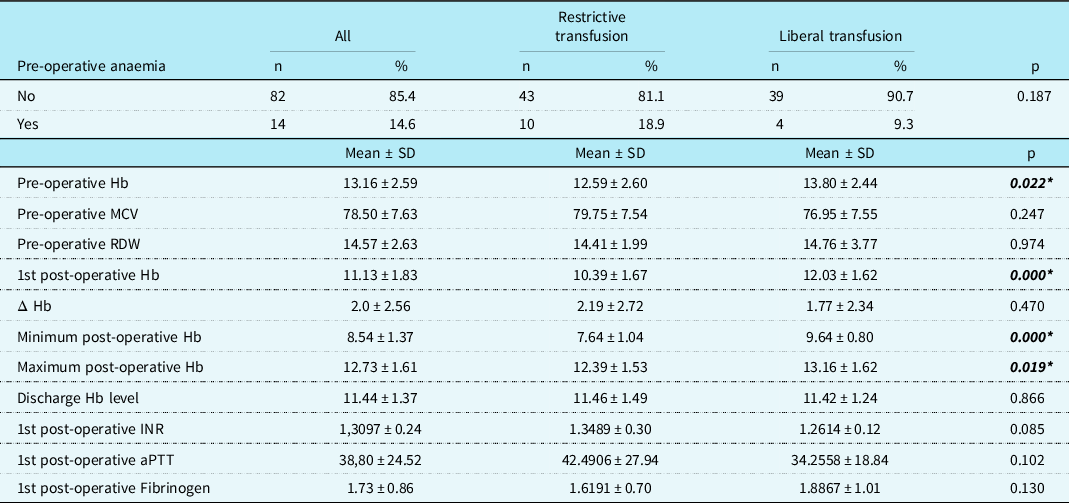
aPTT=activated partial thromboplastin time; Hb=haemoglobin; Δ Hb=pre-operative Hb –1st post-operative Hb; INR=international normalised ratio; MCV=mean corpuscular volume; RDW=red cell distribution width.
*p < 0.05 statistically significant.
Similarly, there were no differences in red blood cell transfusion rates during their inpatient ward stay for each group. Only 1.9% (n = 1) of patients in the restrictive group received red blood cell transfusions, while it was 4.7% (n = 2) in the liberal group (p = 0.43). The results also showed no statistical difference in the 1st post-operative international normalised ratio, activated partial thromboplastin time, and fibrinogen levels between the two groups (p = 0.085, 0.1, and 0.13, respectively).
Table 3 presents the number of blood product transfusions during cardiac intensive care unit follow up. The liberal group received a significantly higher number of red blood cell transfusions than the restrictive group (p < 0.001). The liberal group also received more platelet concentrate transfusions, whereas the restrictive group received more cryoprecipitate (Cryo) transfusions (p = 0.013 and p = 0.029, respectively). The two groups had no significant differences in fresh frozen plasma transfusions (p > 0.5).
Table 3. Number of transfusion of blood products.

Cryo=cryoprecipitate; FFP=fresh frozen plasma; PC=platelet concentrate; RBC=packed red blood cells.
10mL/kg is accepted as one transfusion.
*p < 0.5 statistically significant.
Table 4 displays haemoglobin and lactate levels during and after red blood cell transfusion. The liberal group showed higher levels of haemoglobin at the time of and after the red blood cell transfusion (p < 0.001 and p < 0.001, respectively). There were no differences in lactate levels during and after red blood cell transfusion between the liberal and restrictive groups (p > 0.5).
Table 4. Haemoglobin and lactate levels at the time of and after RBC transfusion of the patients in the study group.

Hb=haemoglobin; RBC=packed red blood cells.
*p < 0.05 statistically significant.
Comparison of clinical features in the restrictive and liberal transfusion groups revealed no differences in the incidence of transfusion reaction, bleeding, re-operation, dialysis, need for extracorporeal membrane oxygenation, acute kidney injury, neurological complications, haematologic and hepatic-related organ dysfunction, coagulopathy, systemic inflammatory response syndrome, and sepsis, as shown in Table 5. Only one patient from the liberal group developed thrombosis due to inherited thrombophilia.
Table 5. Clinical features of the patients in the study group.

AKI=acute kidney injury; ECMO=extracorporeal membrane oxygenation; SIRS=systemic inflammatory response syndrome.
p < 0.05 statistically significant.
Discussion
This study analysed pre-operative and post-operative variables of patients with CHD receiving liberal or restrictive transfusion in the cardiac intensive care unit. The liberal transfusion group had a higher pre-operative haemoglobin level than the restrictive group, while there were no differences in the prevalence of pre-operative anaemia (Table 2). Studies on children undergoing cardiac surgery have demonstrated that pre-operative anaemia is an independent predictor of in-hospital mortality. Reference Cholette, Willems and Valentine7 Nyguen et al. showed that pre-operative anaemia is also related to more red blood cell transfusions and worse clinical outcomes in this patient population. Reference Nguyen, Meng, Berube, Bergstrom and Lam17 Based on the recommendations of the Transfusion and Anaemia Expertise Initiative in 2018, it is crucial to investigate and treat the underlying causes of anaemia before surgery in patients with CHD to prevent unnecessary transfusions during the post-operative period. Reference Cholette, Willems and Valentine7
In our study, the liberal group did not have pre-operative anaemia and had higher levels of pre-operative haemoglobin than the restrictive group. However, they received a higher number of red blood cell transfusions. Patients undergoing surgery for CHD often require transfusions due to factors such as bleeding, consumption coagulopathy, re-sternotomy, and cardiopulmonary bypass-related haemolysis and haemodilution. Reference Guzzetta, Allen, Wilson, Foster, Ehrlich and Miller18 In our study, both groups did not differ regarding cardiopulmonary bypass time, post-operative bleeding, Δ Hb (pre-operative haemoglobin – first post-operative haemoglobin), and re-operation, indicating similar surgical outcomes. Nevertheless, the liberal group received more red blood cell transfusions. This suggests that transfusing patients when their haemoglobin level falls below 9.5 g/dL without assessing their haemodynamic status, liberal transfusion led to a higher number of red blood cell transfusions in this group.
Additionally, the liberal group received more platelet concentrate transfusions. Aluru et al. suggested that the unnecessary red blood cell transfusions may have resulted in dilutional thrombocytopenia, prompting physicians to administer more platelet concentrate. Reference Aluru and Samavedam19 Interestingly, our study found that the restrictive group had a higher number of cryoprecipitate (Cryo) transfusions compared to the liberal group. This difference can be explained by the fact that similar to other European countries, Cryo use was not widespread in our hospital previously; however, its usage has increased since the adoption of the restrictive transfusion strategy. Reference Raphael, Mazer and Subramani8 Furthermore, a recent study by Busack et al. suggested that administering Cryo earlier in the post-operative period can help achieve haemostasis and minimise the need for additional red blood cell transfusions. Reference Busack, Rana, Beidas, Almirante, Deutsch and Matisoff20 In fact, in our study, administering Cryo may have resulted in fewer red blood cell transfusions in the restrictive group.
The mean length of hospital stay in both the restrictive and liberal groups was similar (restrictive 20.36 ± 29.43 [Median: 13] days, liberal 21.09 ± 44.43 [Median: 12] days [p = 0.312]). Despite implementing different transfusion strategies, hospital discharge haemoglobin levels were also similar. Since 2021, our inpatient ward has adopted a restrictive transfusion strategy, and post-operative patients are now provided with multivitamins and iron supplementation. We believe that iron supplementation has contributed to restoring haemoglobin levels in the restrictive group within three weeks. As discussed in the study by Venturini et al., patients restored their haemoglobin levels in twenty days after cardiac surgery with oral iron supplementation. Reference Venturini, Iannuzzo and Lorenzo21
In general, transfusion is commonly employed in patients undergoing surgery for CHD to enhance tissue oxygen delivery. The literature indicates that blood lactate levels, venous-to-arterial CO2 differences, and mixed venous oxygen saturation are important predictors of cardiac output and tissue oxygen delivery. Reference Rocha, Manso and Carmona22,Reference Rhodes, Erwin, Borasino, Cleveland and Alten23 Haemoglobin is critical in oxygen delivery and is often considered the simplest criterion for correcting tissue oxygenation. However, it is also important to optimise other factors, as manipulating haemoglobin concentration alone does not reliably impact oxygen delivery (DO2) or oxygen consumption (VO2). Reference Cholette, Willems and Valentine7 In addition to haemoglobin levels, other determinants of DO2, such as maintaining normal sinus rhythm and /or heart rate control, optimising cardiac contractility, as well as appropriate afterload components of the right and left ventricles, and the use of inotropic agents should be ensured before deciding on a transfusion (except in cases of haemorrhagic shock). Reference Cholette, Willems and Valentine7
In our study, the liberal group had higher minimum and maximum post-operative haemoglobin levels than the restrictive group. However, both groups had similar lactate levels during and after red blood cell transfusion (Table 3). This suggests that transfusion alone cannot be considered the sole parameter for assessing and improving tissue oxygen delivery. Another perspective is that similar lactate levels were achieved with lower haemoglobin levels in the restrictive group, implying that unnecessary transfusions may have been performed in the liberal group.
In our study, the restrictive group received more red blood cells during cardiopulmonary bypass priming than the liberal group. This difference can be explained not by variations in the cardiopulmonary bypass priming technique between the two periods but rather due to the restrictive group having lower pre-operative haemoglobin and body weight levels. Despite there being no difference in priming technique in our study, Wang et al. compared low-priming volume with traditional cardiopulmonary bypass priming and found that the low-priming group had higher haemoglobin levels on the first post-operative day, and low-priming did not adversely affect the post-operative recovery of patients. Reference Wang, Chen and Qiu24
Fortunately, our results did not indicate a difference in transfusion-related complications between the liberal and restrictive groups. However, it is important to remember that the number of transfusions is theoretically related to a greater risk of transfusion-related complications. Unnecessary transfusions can contribute to complications such as coagulopathies, acute kidney injury, pulmonary complications, prolonged duration of mechanical ventilation, and infections. Reference Faraoni, Emani and Halpin25–Reference Kipps, Wypij, Thiagarajan, Bacha and Newburger28 In our study, we found that the need for dialysis and extracorporeal membrane oxygenation, the occurrence of acute kidney injury, neurological complications, haematological complications, hepatic-related organ dysfunction, coagulopathy, systemic inflammatory response syndrome, and sepsis were similar in both groups. Additionally, quality indicators of the cardiac intensive care unit, including mortality, length of stay, and duration of mechanical ventilation, were comparable between the two groups. These findings suggest that similar outcomes can be achieved with lower haemoglobin levels in the restrictive group, making restrictive transfusion a more reasonable strategy.
Limitation
This study has certain limitations that should be conceded. Firstly, the data for each patient were analysed retrospectively, which may raise concerns about data reliability. However, this was minimised as the data in the cardiac intensive care unit were recorded instantaneously into a computer-based system, ensuring that all data were stored in the hospital’s database. Another limitation is excluding patients with single ventricle physiology from the study. These patients require a three-stage operation, and the transfusion approach for each post-operative period of these stages differs. Conducting population-based studies specifically focused on patients with single ventricle physiology would yield more precise results for this specific population. Additionally, neonates under 1 month of age were excluded from this study as their optimal haemoglobin levels differ from the paediatric age group.
Lastly, the assessment of pre-operative anaemia did not involve subdividing the liberal and restrictive transfusion groups into cyanotic and acyanotic CHD subgroups. Although cyanotic patients are known to have higher pre-operative haemoglobin levels than acyanotic patients with CHD, including these subgroups, would have impacted the statistical significance of the outcomes. Reference Faraoni, Meier, New, Van der Linden and Hunt29 Nonetheless, there were no differences in the Risk Adjustment for Congenital Heart Surgery-1 score between the transfusion groups in our study. It should not be forgotten that the effectiveness of using viscoelastic tests such as thromboelastography and rotational thromboelastometry has been investigated by various studies, and these tests are effective in decreasing the need for transfusions. Reference Raphael, Mazer and Subramani8 However, viscoelastic tests are not currently utilised in our clinical practice. Reference Bollinger and Tanaka30
Conclusion
As a conclusion, a restrictive transfusion strategy may be preferable over a liberal transfusion strategy for patients undergoing surgery for CHD. Achieving similar clinical outcomes with fewer transfusions in the restrictive group provides promising evidence for future investigations.
Acknowledgements
The authors gratefully acknowledge the statistical assistance of Canser Boz, PhD from Istanbul University, Faculty of Health Science.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.
Ethical standards
This study was approved by Koc University Ethics Committee on Human Research (Approval Number: 2022.259.IRB1.100).








