Transcatheter closure of atrial septal defects involves the use of pre-formed devices and delivery systems, developed based on standard cardiac and extra-cardiac anatomy. However, in the presence of complex septal morphology or extra-cardiac factors like severe scoliosis or diaphragm paralysis, the procedure may become more challenging, and pre-formed sheaths and devices thought to facilitate the implantation might turn into a problem to address.
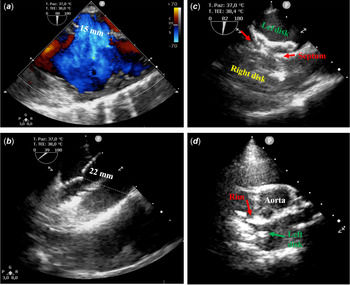
Few days before the publication of the paper by Herron and Kobayashi, Reference Herron and Kobayashi1 we have experienced a similar issue. A 12-year-old female patient with severe scoliosis underwent percutaneous closure of a 22 mm atrial septal defect using a Gore Cardioform atrial septal defect device® under trans-oesophageal echocardiography (TOE) guidance (Fig 1). Trans-oesophageal echocardiography showed a central defect with floppy posterior rim and a right-convex dorsal scoliosis, QP/QS was 2.1, mean pulmonary artery pressure 17 mmHg, and pulmonary vascular resistance 0.7 WU*m2. Initially, a 37 mm Gore Cardioform atrial septal defect device was delivered; however, the device positioning proved to be challenging due to the relatively small left atrium and the floppy posterior rim. The device was then removed and was upsized to 44 mm Gore Cardioform atrial septal defect device. This time, the device conformation and positioning were relatively straightforward. Trans-oesophageal echocardiography displayed no residual shunt despite the significant tension causing distortion of septal anatomy. Based on these findings, we decided to release the locking loop. Following this manoeuvre, strong tension still persisted on the device, even though no shunt was detected. As a consequence, we decided to remove the retrieval cord in order to relieve the tension and complete the alignment of the device with the septum. Unfortunately, sudden release of tension resulted in device malpositioning. We attempted to retrieve the device using a 16 Fr CheckFlow Performer Long Sheath (Cook®) and a 15 mm Andra® snare catheter. Despite being easily catchable by the locking loop, the angle between inferior vena cava and right atrium made it impossible to obtain an adequate alignment between long sheath and device in order to retrieve it. As a result, the device embolised in the tricuspid valve. Finally, the device was stabilised in the right atrium by snaring the locking loop and the patient was sent for surgical removal of the device and atrial septal defect patch closure.
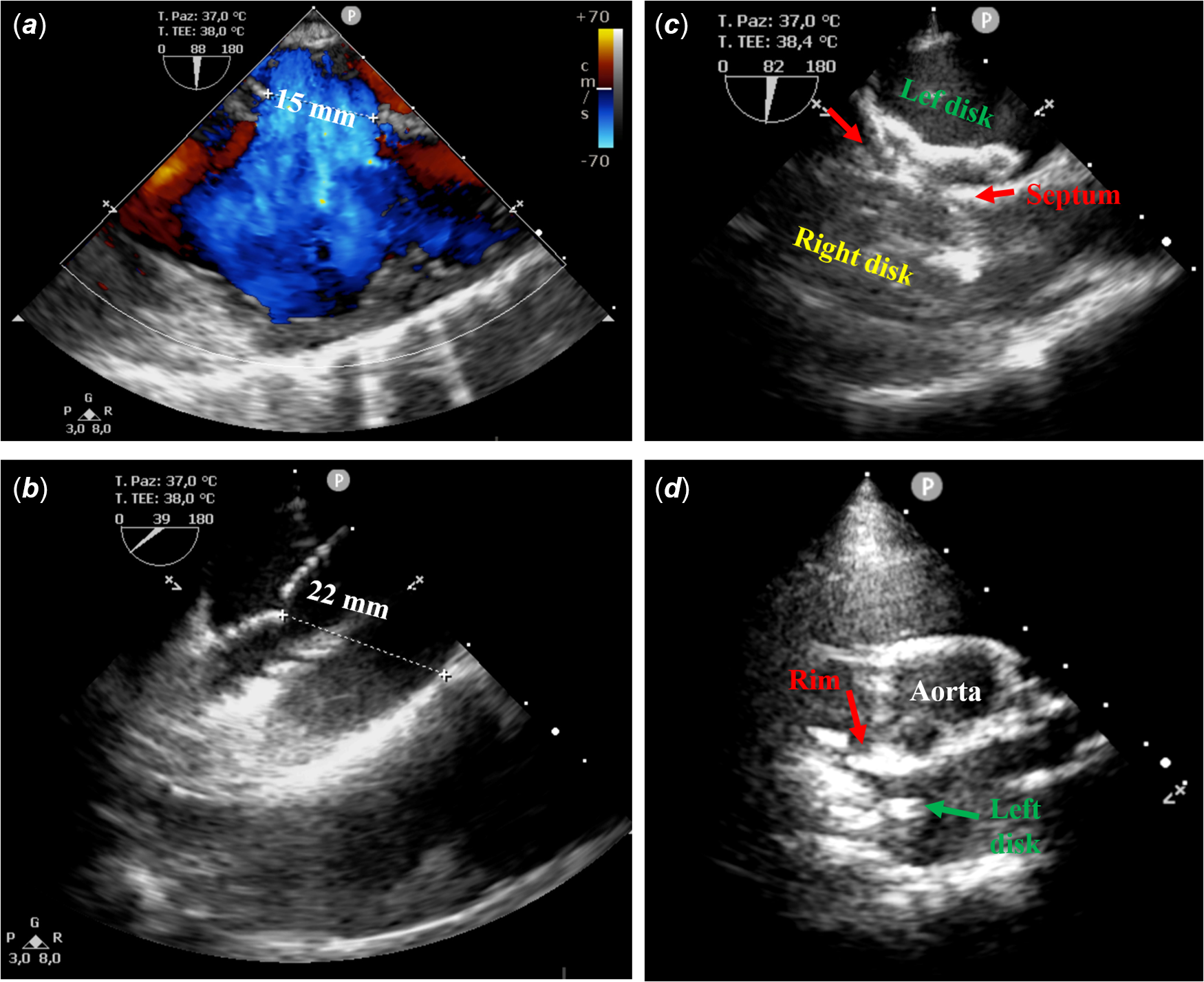
Figure 1. Intra-procedural trans-oesophageal echocardiography. ( a ) Baseline atrial septal defect diameter, 15 mm. ( b ) Stretched diameter at the static sizing (22 mm). ( c ) Device after locking loop closure. ( d ) Device prolapse after safety cord removal.
In conclusion, the Gore Cardioform atrial septal defect device is a new soft and conformable device, with optimal adaptability to different atrial septal defect anatomies. In Europe, it is available since January 2020. The advantage of this new self-centring device consists in the possibility to oversize the device in order to overcome atrial septal defect anatomical issues and to cover side holes. Reference Santoro, Castaldi and Cuman2,Reference Sommer, Love and Paolillo3 Unfortunately, the stiffness of the delivery system might represent a pitfall in some challenging anatomies. As reported by Herron and Kobayashi, Reference Herron and Kobayashi1 the use of a Mullins sheath to support the device and preventing the prolapse during the release may overcome this limitation in some circumstances (i.e., severe scoliosis or diaphragm paralysis). In case of device embolisation, the use of the Mullins sheath might also improve the alignment between the device disk and the CheckFlow Long Sheath in order to facilitate the device retrieval. Alternatively, a second venous approach (e.g., right jugular vein) may be used to introduce a second long sheath, achieving better alignment to the device or to proceed with a double snaring technique (veno-venous) in order to elongate more easily the device from both sides, allowing its capture and successful retrieval even in challenging situations.
Conflict of interest
None.