Introduction
Norovirus is the leading cause of gastrointestinal illness in humans worldwide [Reference Harris, Iturriza-Gomara and O'Brien1–Reference Kirk3]. Outbreaks are common and happen through several means of transmission (food, water, person-to-person and environment); food and waterborne outbreaks occur frequently [Reference Murphy4, Reference O'Brien5]. They are often followed by person-to-person transmission in households [Reference de Laval6]. Norovirus is also the culprit of large-scale person-to-person outbreaks in health care settings and other institutions [Reference Bonifait7, Reference Friesema8]. In food facilities, the means of transmission has been established to be either an infected, often symptomatic food handler [Reference Franck9, Reference Iversen10] or fresh produce (or a similar product) [Reference Callejon11, Reference Baert12] that has been contaminated with the irrigation water in the growing process [Reference Kokkinos13, Reference Mok, Barker and Hamilton14]. However, a number of foodborne outbreaks remain obscure in terms of the source of the infection [Reference Rumble15]. Ice [Reference Parshionikar16–Reference Cheng18] and drinking-water [Reference Braeye19, Reference Jalava20] outbreaks are also common, often involving multiple pathogens from wastewater leaking to drinking water [Reference Barker21–Reference Sano23]. Ice usually becomes contaminated through wastewater leakage to drinking water somewhere along the water distribution line [Reference Parshionikar16–Reference Cheng18]. Airborne transmission is possible through aerosols generated by vomiting or flushing the toilet [Reference Bonifait7, Reference Lopman24, 25]. However, although norovirus has been detected in toilet surroundings aerosols [Reference Verani, Bigazzi and Carducci26], spreading through aerosolisation and vaporisation of faecal waste water has been found to be relatively uncommon [Reference Marks27–Reference Zhang29]. In Europe, norovirus infections were at an elevated level in Europe at the beginning of 2017 [Reference Mayor30]. Recently circulating strains have become more resistant and increasingly human-to-human transmissible [Reference Matsuyama, Miura and Nishiura31].
A notification regarding gastroenteritis among a group of Christmas party attendees from company A was received by the City of Tampere food authorities on 12 December 2016. After the party, which took place on 9 December and was attended by 66 persons, several had experienced severe gastrointestinal illness. The party was composed of four parts: going to the cinema, an ‘amazing race’ which took place outdoors, dinner in restaurant C and further celebrations at the nightclub D. Based on the information from the party goers from company A, all participants who fell ill had attended the dinner in restaurant C; no food was served at the other functions (excluding popcorn at the cinema). Soft drinks were also served at the cinema and a 2 dl wine bottle and another alcoholic drink were offered during the outdoor activities. A request was made to restaurant C inquiring about other possible diners. A group from company B had also organised a Christmas party at restaurant C with an identical menu for 88 of its employees on 10 December 2016. Some gastrointestinal illness was also reported among company B attendees after consulting them. Non-buffet dinner was also available for other visitors and some of the non-buffet diners reported suffering from gastrointestinal illness symptoms as well, as was learned after contacting them by phone. As contacting all of them was not possible and non-buffet menus were variable, they were excluded from the study. According to local health authorities, no excess of gastrointestinal illness had been detected elsewhere in the area.
The aim of this study was to determine the magnitude of this outbreak, identify and control the source and prevent further illness in the community.
Methods
Epidemiology
Participants were enrolled by contacting the companies A and B who provided a list of participants for the events. Participants were contacted by email through a company contact person. A formal, standard cohort web-based study was conducted with Christmas party attendees from companies A and B, but excluded restaurant personnel and non-buffet diners. Dose-response questions were asked to gather information on all foods and drinks consumed and how much was eaten, with a focus on fresh produce, water and ice on drinks. Dose-response on drinks was considered with increasing consumption of ice (whether separately in a drink or by a jug of water). A case was defined as a person who experienced diarrhoea, vomiting or two other symptoms following attending a Christmas dinner party with company A or B on the 9 or 10 December 2016 at restaurant C. The descriptive analysis on symptoms was done on the entire cohort. The results were analysed using LibreOffice Calc (Linux), Excel® and R (Cran) statistical programs. For the risk ratios, profile-likelihood-based confidence intervals were used.
Hygienic inspections and sampling from the restaurant's kitchen premises
An inspection was made of restaurant C on 13, 19, 22, 23 and 27 December 2016. During these inspections, hygienic practices were reviewed, and samples were taken for study. Also, buffet menus for companies A and B were confirmed as well as food on the menu for non-buffet diners those evenings. Hygienic instructions were given regarding ice cube facilities and the air ventilation valve of the sewage system located adjacent to ice cube machines (half a metre above) was checked.
Patient samples
Those who reported illness symptoms were recorded by the Infectious Diseases Control Unit and requested to submit a faecal sample. Faecal samples were also requested from three kitchen personnel after the notification of the outbreak. The faecal samples were analysed for major bacterial pathogens (salmonella, campylobacter, yersinia and shigella) by culture and viral pathogens (norovirus, sapovirus) by PCR in Fimlab (local diagnostic laboratory) in Tampere and the National Institute for Health and Welfare (THL).
Water, ice and environmental samples
No leftover foods were available for sampling. Ice and water were implicated as vehicles only from the cohort study results and samples were taken subsequently. Ice samples taken on 19 and 22 December 2016 were analysed for heterotrophic total bacterial counts incubated in +22 °C, coliforms and E. coli according to standardised methods. The air ventilation valve and its outer surface were sampled for a viral analysis on 27 December 2016. The tap water samples taken on 19 December 2016 and a flush sample from the air ventilation valve cap taken on 27 December 2016 were analysed for norovirus and sapovirus. A 2 l tap water sample was obtained using the adsorption-elution method as previously described [Reference Kauppinen, Pitkänen and Miettinen32]. Surface samples were collected with an EnviroSwab (3 M) and swabs were eluted with 10 ml of PBS. Mengovirus was added as a process control to the water and surface samples. Viral nucleic acids were extracted from 200 µl of the water concentrate and 2.5 ml of the surface samples using the High Pure Viral RNA and High Pure Viral Nucleic Acid Kits (Roche Molecular Biochemicals Ltd), respectively. Noroviruses were detected using primer-probe sets described in Kauppinen et al. [Reference Kauppinen, Pitkänen and Miettinen32]. The real-time-quantitative polymerase chain reaction assay was carried out as previously described for noroviruses [Reference Kauppinen and Miettinen33] and for sapoviruses [Reference Svraka34].
Results
Epidemiology
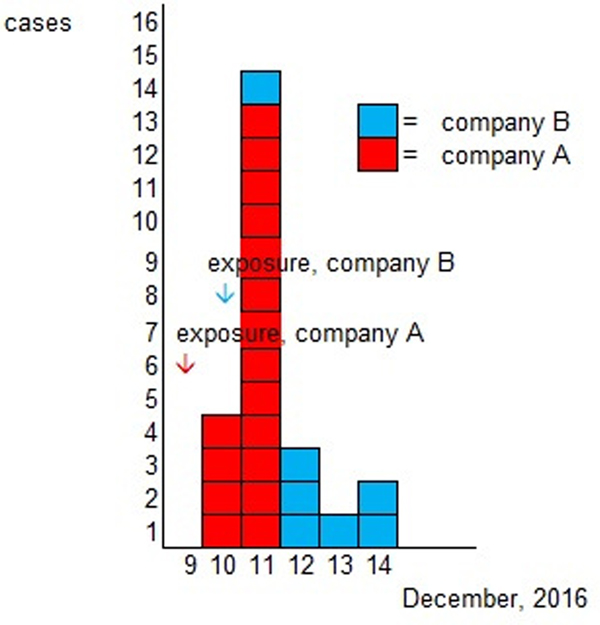
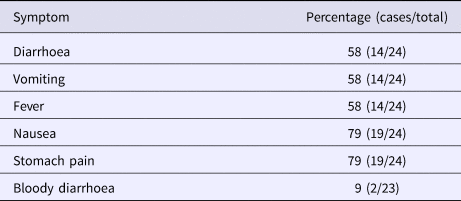
Altogether 82% (54/66) of the Christmas party attendees from company A and 55% (48/88) of the attendees from company B responded to the study. A total of 91 persons were enrolled in the study after 10 persons who had been travelling abroad (outside the Nordic countries) and one duplicate was excluded. The case definition was fulfilled by 26% (24/91) of the respondents, 17 (71%) from company A and 7 (29%) from company B. One self-reported vomiting due to a hangover was excluded from cases. As the menu was the same for both parties and the ingredients used in preparing the meals were from the same batch, the statistical analyses were combined. Of the respondents, 37% (34/91) were men. The characteristic symptoms within the cohort were diarrhoea (16%, 14/90), vomiting (15%, 14/91) and fever (16%, 15/91); general symptoms of nausea (23%, 21/91) and stomach pain (22%, 20/90) were reported as well. Two cases (2%, 2/88) were reported to have bloody diarrhoea. The distribution of onset of symptoms is presented in the epicurve, in Figure 1 and the symptoms for cases in Table 1.
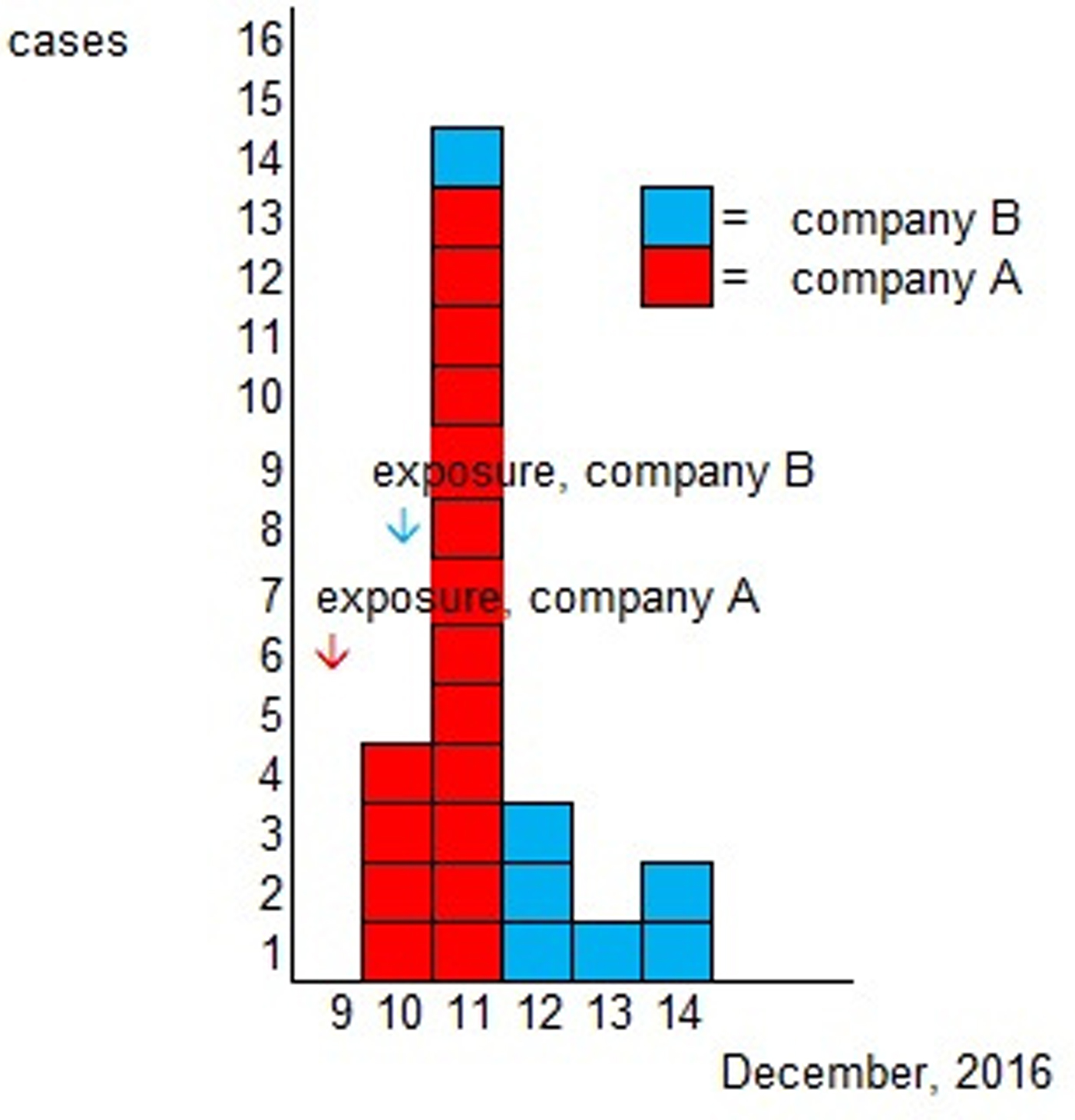
Fig. 1. Epidemic curve according to onset of illness.
Table 1. Distribution of symptoms in case patients
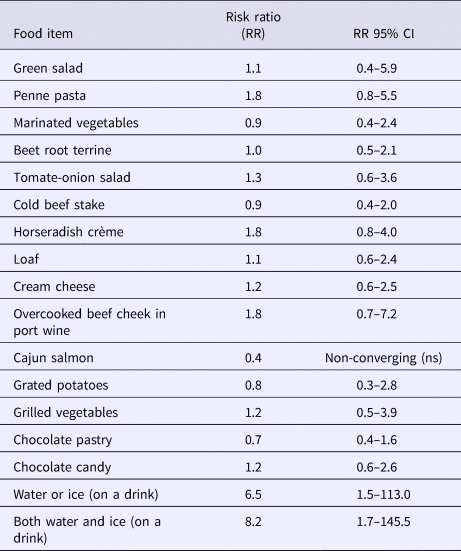
All foods and common drinks were analysed, Table 2. The water and ice cubes were combined as one response and analysed by dose response (0 = no ice, no water or only water (company B)); 1 = water (company A) or ice in any drinks (company A or B); 2 = water and ice in any drinks (company A), as the water served to attendees from company A included both water and ice. Those attending the party from company B were able to drink water, ice or both. The risk of becoming ill was 6.5 times higher if water or ice was drank compared with the non-exposed. The risk further increased to being 8.2 times higher if both were consumed compared with the non-exposed. There were 17 persons not exposed to ice or water and 15 of these were from company B. Company B attendees consumed a large amount of alcohol, so it is likely that even most of these non-exposed had ice in some drinks but recall bias might have been severe. Other foods were not significant.
Table 2. Foods and drinks consumed in restaurant C during the weekend 9 and 10 December 2016 with RR (risk ratios) and respective 95% confidence intervals
Patient samples
From the patient samples, three cases of norovirus genogroup I was diagnosed. In one of these cases, both norovirus and sapovirus were detected, as was in a symptomatic family member of the patient. Norovirus genogroup I was detected in one of the asymptomatic restaurant serving staff members. However, the positive staff member was on duty only on Saturday, 10 December, drank soft drinks with implicated ice while on duty and, therefore, was considered an unlikely source of the outbreak. None of the staff members experienced gastrointestinal illness prior to or after the incident.
Water, ice and environmental samples
Restaurant kitchen
The hygienic level was reasonable, but some requests on improving hygiene were made and some equipment was slightly out of date. The personnel was experienced and they had recorded notes according to standard. No clear divergences in practices were noted and some routines were updated. In the ice cube room, there were two ice cube machines, one crushed ice machine, a washing machine and a drier (for employees work clothes), a freezer, a refrigerator, two large beer tanks and barrels of alcoholic drinks, Supplementary video S1 (taken on 27 December 2016). A large amount of extra equipment was stored in the room space, however, that was not considered a direct cause of the problem. During the inspection on 19 December, when the ice cube machines were inspected for the first time, they contained plenty of ice. There were no deviations in the hygiene of the ice cube machines or devices used to pick up the ice in the visual inspection. A visually clean scoop, stored on the top of the ice machines was used to gather the ice. The washing machine and dryer situated in the same room may have contributed to air movements. According to restaurant C employees, the laundry machines were used frequently and often during the peak time of weekends. There were a regular cleaning and maintenance protocol, which had been followed. Restaurant C was found to have conducted all recommended and agreed upon construction works during subsequent inspections. The air ventilation valve pipe and its connecting ventilation sink line was transferred to another room on 28 December, an adequate cleaning and disinfection process was carried out and completed on 29 December in the ice cube room. The completion of the recommended construction was checked by the construction unit of City of Tampere on 18 January 2017.
High levels of heterotrophic bacteria (estimated levels 7000 and 7500 cfu/ml, detection limit <3000 cfu/ml) were detected on ice cube samples taken on 19 December. No norovirus was detected in the tap water taken from the tap in the room that held the ice cube machines. The ice tested negative for the faecal indicators E. coli and coliformes. Based on these results further samples were taken on 22 December from the crushed ice machine (a third machine used for ice preparation). This sampling detected heterotrophic bacteria of 140 cfu/ml and the water sample from the water pipeline system used for delivering water to the ice cube machines was negative for norovirus. During the inspection on 23 December, a dysfunctioning air ventilation valve was implicated as the most likely cause of the outbreak (see Figs 2 and 3). The cover was loose and the sealant had not been properly installed. Three surface samples from the air ventilation valve tested negative for norovirus and sapovirus.

Fig. 2. The implicated white coloured air ventilation valve and its grey connecting ventilation sink behind the ice cube machine.

Fig. 3. Black smudge on the right-hand side on the neck of the valve where the valve was leaking.
Discussion
The evidence suggests that this viral gastrointestinal outbreak among Christmas party goers was caused by contaminated ice cubes at a restaurant in the Pirkanmaa region of Finland. The ice cubes were most likely contaminated by air due to a leaking air ventilation valve located in the same room. The ice cube machines were closable, clean by visual inspection and the scoop was clean, although not in the appropriate storage location. Tap water tested negative for norovirus and was in closed tube system before reaching the ice cube machines, therefore considered an unlikely source. The washing machine and drier may have contributed to the circulation of the air in the room space. The detection of two pathogens in patient samples indicated early on in the investigation that there may have been faecal contamination on food or drink. It is not common to find two or more pathogens unless there is sewage contamination.
The relatively severe course of illness (fever and some bloody diarrhoeal cases) was in line with the causative agents detected. This particular season was found to have a high rate of norovirus infection overall in the UK and presumably, the same phenomenon took place all over Europe [Reference Mayor30]. Finnish national surveillance data from THL indicated a severe season (data not shown).
The cohort study clearly implicated the role of the ice cubes as a vehicle for the outbreak. This was further supported by the high levels of heterotrophic bacteria found on the tested ice cubes [Reference Mako35]. As the usage of ice cubes was frequent and the machinery visually clean and regularly washed and disinfected, only an outside source could explain the high counts observed. Furthermore, normal tap water was used for the preparation of the ice cubes. As there were no leaks in the water delivery system, nor construction works, an alternative source was sought. An air ventilation valve and its connecting ventilation drain were detected in the ice machine room after cleaning the cardboards away from the top of the machines that prevented visualisation during the first inspection. Even though the evidence suggests that the ice cubes were most likely contaminated by air leaking from the faulty air ventilation valve, we only have circumstantial evidence, therefore not proving causality. More outbreaks caused by similar mechanism should be described to confirm this mode of transmission on a larger scale.
The role of the air ventilation valve and its connecting ventilation drain is normally to take in the air when a toilet is flushed. As the air ventilation valve was leaking air to the room, aerosols were easily released into the room space. Surprisingly, there were no odd odours observed in the ice machine room despite it being directly connected to wastewater through the sink system and toilets. According to the constructor of the water pipeline, the air ventilation valves and their connecting ventilation sinks are routinely built up to the roof. However, some construction difficulties and heavy usage of toilets prevented this form be done in this building. According to construction authorities, this is a relatively common practice if building difficulties arise, there is heavy usage of toilets or if there are cost restraints. The room space was originally used for dishwashing but had been changed over the years to function as a space for the ice machines and storage.
Leaking air ventilation valve was the most probable cause of this outbreak. This represents a novel means of transmission but may not be uncommon in other settings where norovirus outbreaks are common, including food facilities, hospitals and elderly homes. Epidemiologists and other experts on the prevention and control of infectious diseases have not previously been aware of this type of possibility in the water pipelines. There are virtually no norovirus transmission references to our knowledge in the literature involving this type of mode of transmission. The norovirus genogroup I detection from case-patients further suggested the route of transmission as waterborne as genogroup I is often associated with food or waterborne transmission [Reference Matthews36].
However, the leaking air-ventilation valve mechanism resembles the mode that was described in the outbreak of SARS that occurred in the Amoy Gardens housing block in Hong Kong in 2003 [Reference Hung37]. Dried-up U-traps in floor drains were concluded to be the main transmission route within Block E, the most affected of the buildings. We have identified this type of air ventilation valve and the connected ventilation sinks in food facilities, albeit in acceptable, non-high hygiene areas (data not shown). We have also noticed that drains have been largely neglected in routine inspections and that leaking U-traps are a common feature in so-called high hygiene areas of food processing facilities (data not shown). There has been some modelling work done on the spread of SARS/norovirus in housing and hospital drainage systems [Reference Gormley38, Reference Gormley39]. Norovirus infection can be a huge burden in a community and a major reduction in the illness may be able to be obtained if even a modest proportion of cases can be prevented. A recently published paper suggests that a salmonella outbreak spread from contaminated drains and leaking U-traps [Reference Mair-Jenkins40]. However, as the authors of that paper did not mention why cases only occurred in carvery meat eaters and did not mention the sampling of the carvery (only significant exposure in the case-control study), the source's validity remained somewhat uncertain. All food and other hygienic facilities should be checked for the location and function of air ventilation valves, especially if norovirus outbreaks are detected.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095026881800314X.
Author ORCIDs
K. Jalava 0000-0001-9106-3186.
Acknowledgements
We thank Marjaana Leinonen and Maija-Liisa Rantala for excellent assistance.
Conflict of interest
None.