INTRODUCTION
It has been shown that HIV leads to a decline in muscle function and reduced physical activity [Reference Kusko1, Reference Kosmiski2]. In sub-Saharan Africa, many HIV patients are relying on labour-demanding jobs in the informal sector with no job security or compensation for lost income. Maintaining physical capacity and an adequate activity level is thus of crucial importance for their livelihoods. In African countries, the impact of HIV might be further aggravated by widespread food insecurity and inadequate energy intake [Reference Gillespie3]. Studies of Kenyan tea pluckers [Reference Fox4] and analyses of financial records from private companies across Africa [Reference Rosen5] have shown a decline in productivity and substantial loss of income in workers with HIV. However, only few studies have made direct measurements of energy expenditure in HIV patients. These have mainly been conducted in high-income settings and have reported normal levels of total energy expenditure (TEE) in spite of elevated resting energy expenditure (REE), indicating a reduction in physical activity energy expenditure (PAEE) [Reference Kosmiski2]. One study observed an inverse relationship between viral load and physical activity in HIV patients [Reference Bopp6]. A study of tuberculosis (TB) patients in Tanzania found that HIV co-infection was associated with substantially lower levels of physical activity [Reference Faurholt-Jepsen7] but, so far, no study has focused on physical activity and its predictors in a cohort of African HIV patients.
As part of a large nutritional supplementation trial in HIV patients in an urban setting of South-West Ethiopia, we used objective measurements of habitual physical activity and physical capacity. The objectives of this paper are to (1) describe habitual physical activity and physical capacity in adult HIV patients at initiation of antiretroviral treatment (ART), (2) assess the role of HIV severity and anaemia, and (3) assess the role of nutritional status, food security and energy intake on functional outcomes.
METHODS
Study setting and population
In Ethiopia, ~1·2 million people are living with HIV, which has an estimated prevalence of 1·5% (urban 4·2%, rural 0·6%) [8]. Jimma and Agaro towns have populations of about 150 000 and 30 000, respectively [9] and are located in a generally food-secure area of the Oromia region. However, studies have shown that the majority of HIV patients are affected by food insecurity [Reference Tiyou10, Reference Olsen11]. The data presented here were collected in a cross-sectional study nested in a trial of HIV patients commencing ART conducted during 2010–2013 at Jimma University Specialized Hospital (JUSH), Jimma Health Centre (JHC) and Agaro Health Centre (AHC). Inclusion criteria were age ⩾18 years, eligible for ART initiation, not pregnant or lactating, residing within 50 km of Jimma or Agaro towns and written consent to participate. The criteria for ART initiation were CD4 ⩽200 cells/μl irrespective of clinical symptoms, CD4 ⩽350 cells/μl if clinical stage III, or WHO clinical stage IV irrespective of CD4 [12]. For logistical reasons, it was not possible to include all patients in physical activity monitoring. Patients recruited at JUSH and JHC were invited for physical activity monitoring, while patients recruited from AHC were assessed by a mobile study team, which did not have resources to make the additional visits needed for physical activity monitoring. Grip strength data was obtained from patients at all sites. Furthermore, grip strength data was available from an HIV-negative reference group (n = 100). The reference group was recruited from the Voluntary Testing and Counselling service at JUSH and matched on sex, age and body mass index [BMI; weight (kg)/height (m2)] group with the last 100 HIV patients recruited. Background information including demographic data, anthropometry and energy intake were collected for all participants.
Data collection
Demographic data were collected using a structured questionnaire administered in local languages by trained study staff. Height and weight were measured with the participant barefoot and wearing minimal clothing, using calibrated stadiometers and scales, respectively. BMI was categorized as <16, 16–18·5 and >18·5 for analyses to indicate severe underweight, underweight, and normal weight, respectively [13]. Mid-upper arm circumference (MUAC) was measured at the midpoint of the left arm between the acromion process of the shoulder and the olecranon process of the ulnar bone. MUAC was categorized as <21 cm or ⩾21 cm for analyses to indicate low or normal values [14]. Triceps skinfold thickness (TSF) was measured at the midpoint of the back of the left upper arm using a Harpenden skinfold caliper (Baty International, UK). Corrected arm muscle area (CAMA) was derived from measures of TSF and MUAC and corrected for bone area using standard formulae [Reference Heymsfield15]. Food security status was assessed using the Household Food Insecurity Access Scale (HFIAS) [Reference Coates, Swindale and Bilinsky16]. The HFIAS includes nine items addressing the occurrence of food insecurity within the last 30 days. The scale was used to classify participants' households into four levels of food insecurity according to a scheme modified from the HFIAS protocol and validated for use in Ethiopia [Reference Maes17]. Energy intake was assessed from a recall of the previous 24 h using a pre-coded chart and a locally developed recall kit with household utensils and models of Ethiopian dishes. Energy content was based on local food composition tables [18]. The recall was also used to assess intake of alcohol, tobacco and khat.
Habitual physical activity was expressed by PAEE and activity intensity (sedentary and moderate/vigorous). Physical capacity was expressed by heart rate (HR) economy, sleeping heart rate (SHR) and grip strength. Physical activity was measured using a combined uniaxial accelerometer and HR sensor (Actiheart, CamNtech, UK) as described elsewhere [Reference Brage19]. The monitor was fitted on the upper left side of the chest on two ECG electrodes (Unomedical A/S, Denmark), placing one electrode in the centre of the chest and the other laterally. HR was calibrated using a 250 m self-paced walk test followed by 2 min rest. Individual variation has been shown in the relationship between HR and energy expenditure, suggesting that estimates of habitual physical activity should be adjusted for individual indicators [Reference Brage20]. We therefore adjusted activity estimates for a proxy of individual fitness calculated as the walking distance per heart beat above rest (HR economy). After the walk test, the monitor was set up for a 4-day recording during habitual living, collecting data every 15 s. Participants were informed about the purpose and functions of the monitor and requested to wear it continuously while continuing with their habitual activities. Data were cleaned and post-processed using a Gaussian robust process regression model to handle noisy HR data [Reference Stegle21]. Prolonged recording time (>60 min) with non-physiological HR and no movement was marked as non-wear time. Individual recordings were included in analyses if they contributed ⩾24 h of valid monitor-worn activity data. SHR was included as an indicator of physical capacity as it is known to be associated with individual fitness. SHR was determined from days with complete recordings which included a night of sleep. Activity level and HR was modelled using branched equation modelling for the estimation of average daily PAEE [Reference Brage22]. The estimation of activity intensity was determined using group calibration equations for both accelerometry and HR [Reference Brage20, Reference Christensen23]. Sedentary activity was described as the percentage of time spent ⩽1·5 metabolic equivalents (MET) while moderate/vigorous activity was described as percentage of time spent >3 MET. TEE was derived as the sum of PAEE, predicted REE and diet-induced thermogenesis (10% of TEE). Prediction of REE was based on sex, age, height and weight using the Oxford equations [Reference Henry24] and adjusted +10% according to the increase of REE seen in HIV patients [Reference Kosmiski2]. Physical activity level (PAL) was determined as TEE/REE. At the completion of recordings, a locally developed 24-h recall questionnaire was used to collect information about the types of activities participants had engaged in. Grip strength was determined using a digital grip dynamometer (Takei Scientific Instruments Co., Japan). The measurements were performed twice each for the right and left hand alternately. The mean of the highest right and left hand value, respectively, was recorded. This procedure was repeated and the highest value was used for analyses.
Whole blood specimens were collected in EDTA tubes. CD4 counts were determined with flow cytometry (Fascount, Becton Dickinson, USA) and categorized as <100, 100–200 and >200 cells/μl for analyses. Plasma was kept at −80°C before HIV-1 viral load was quantified using a commercial PCR assay (RealTime HIV-1, Abbott Laboratories, USA) with automated extraction (m2000 Real Time System, Abbott Laboratories). Haemoglobin was determined using an automated haematology analyser (Sysmex KX-21N, Japan). Haemoglobin <125 g/l for women and <135 g/l for men defined anaemia, as standard cut-offs were adjusted +5 g/l to account for the 1700 m altitude of the area [25]. Anaemia was further categorized as grades 1 and 2, by dividing at 1 s.d. below the anaemia cut-off of women and men, respectively [grade 1: 105–125 g/l (women), 117–135 g/l (men); grade 2: <105 g/l (women), <117 g/l (men)].
Information regarding WHO clinical stage of HIV, opportunistic infections (OIs) and other illnesses were extracted from the patients' records. Patients were considered TB co-infected if they were receiving TB treatment at study inclusion. Patients with herpes zoster, pneumonia, Kaposi's sarcoma, toxoplasmosis, chronic fever, chronic diarrhoea, or wasting syndrome at the time of study inclusion were considered as having other OI or illness.
Statistical analysis
Data were double entered and validated using Epidata (EpiData Association, Denmark) and analysed using Stata/IC version 11.2 (StataCorp LP, USA). Differences in means and proportions between men and women were tested using t test and χ 2 test, respectively. Normal probability plots were used to assess normality of continuous variables. Log-transformation was used to achieve normal distribution of PAEE [log(PAEE)] and viral load [log(1 + copies/ml)]. PAEE, sedentary activity, moderate/vigorous activity, HR economy, SHR and grip strength were used as dependent variables in linear regression models. The role of each potential predictor was assessed in individual models. Additional models included both HIV and nutritional status to determine if malnutrition was a mediator for the effect of HIV on functional outcomes or vice versa.
By comparing unadjusted and adjusted models, demographic variables (sex, age, education) were tested for their potential role as confounders and regression models were adjusted accordingly. Interactions between each of the potential predictors of functional outcomes and sex or age, respectively, were tested in all models. Furthermore, interactions between HIV and nutritional status were assessed. Since SHR is influenced by individual fitness as well as disease status, estimates of SHR were adjusted for HR economy in secondary analyses to estimate the effect of HIV indicators not explained by differences in individual fitness. In addition, a potential confounding role of anaemia was tested for all other outcomes. Associations with P values <0·05 were considered significant. The 24-h recall data were used to describe types of activities and their prevalence in study participants.
Ethical approval
Written and oral information about the purpose and methods of the study were given to all eligible participants by the study staff before written informed consent was obtained. Ethical permission was obtained from the Ethiopian National Health Research Ethical Review Committee and Jimma University Ethical Review Board. A consultative approval was obtained from the Danish National Committee on Biomedical Research Ethics.
RESULTS
A total of 453 HIV patients were screened between July 2010 and August 2012. Of these, 348 (77%) were enrolled. Participants were younger (32·9 vs. 37·0 years, P = 0·001) and had lower education (21 vs. 45% with secondary school or higher, P < 0·001) than those not enrolled, while BMI and demographic characteristics were similar. Of 270 eligible patients at JUSH and JHC, complete data on physical activity were obtained for 243. The mean of physical activity monitoring was 4·2 (range 1·0–9·3) days. Grip strength data was obtained for 343 patients at JUSH, JHC and AHC.
The characteristics of study participants are presented in Table 1. Mean±s.d. age was 32·9 ± 8·8 years and two-thirds of participants were women. The majority were working in the informal job sector, mainly as daily labourers (26%), traders (12%) or farmers (5%). Mean±s.d. BMI was 19·1 ± 2·6. Almost half of the participants were underweight (BMI 16–18·5 in 34% and BMI <16 in 9%), whereas overweight was uncommon with BMI ⩾25 in only 3%. Mean±s.d. reported energy intake was 6·1 ± 2·7 MJ/day. Two-thirds of participants were affected by either moderate or severe food insecurity. Household food security status was associated with energy intake which was 7·2 ± 2·5 MJ/day in food-secure participants, while it was 6·5 ± 3·0, 6·3 ± 2·8 and 5·2 ± 2·4 MJ/day, respectively, in participants from households with mild, moderate and severe food insecurity. Very little smoking (3%), alcohol intake (2%) and khat use (5%) was reported and this was therefore not explored further. Mean±s.d. CD4 count was 187 ± 110 cells/μl, with CD4 <100 cells/μl in 22% and CD4 100–200 cells/μl in 39%. Almost half of the participants were anaemic grouped as either grade 1 (32%) or grade 2 (15%). The characteristics of participants from JUSH and JHC who had physical activity monitored were similar to participants from AHC in terms of sex, age, WHO stage and BMI. However, the physical activity subsample had a higher education level (25% vs. 12% with secondary school or higher, P = 0·007) and lower energy intake (5·8 vs. 6·7 MJ/day, P = 0·004) than participants from AHC.
Table 1. Characteristics of HIV patients at initiation of antiretroviral treatment (n = 348)
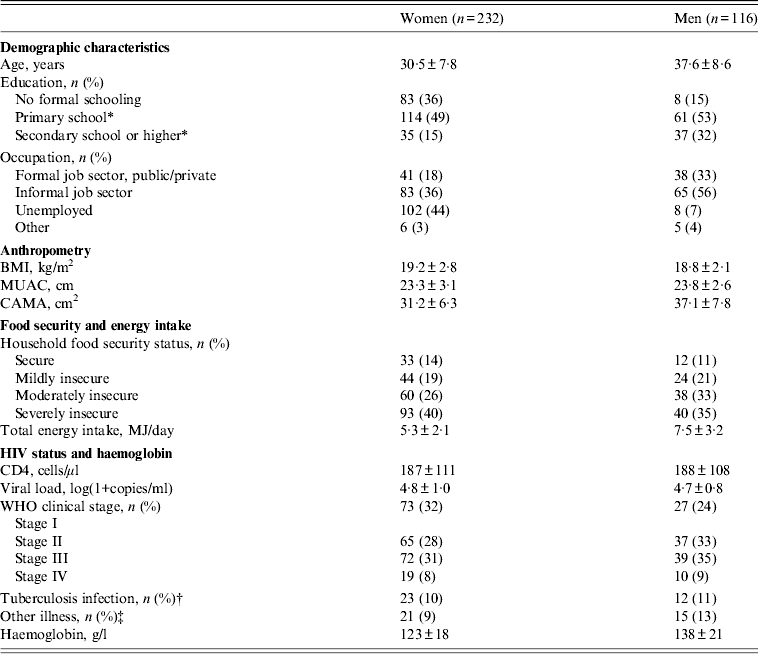
BMI, Body mass index; MUAC, middle upper arm circumference; CAMA, corrected arm muscle area.
Data are mean±s.d. unless otherwise indicated.
* Includes participants with some/completed level of education.
† Includes participants receiving tuberculosis treatment at the time of inclusion.
‡ Includes participants with herpes zoster, pneumonia, chronic fever, chronic diarrhoea or wasting syndrome at the time of inclusion.
Crude estimates of walk test data, physical activity, energy expenditure and physical capacity for women and men, respectively, are presented in Table 2. Median PAEE of women and men were 23·5 [interquartile range (IQR) 15·5–33·9] and 38·0 (IQR 25·2–54·7) kJ/kg per day, while mean TEE was 155·6 [95% confidence interval (CI) 152·8–158·5] and 178·6 (95% CI 172·7–184·5) kJ/kg per day, respectively. Men spent almost twice as much time as women in moderate/vigorous activity (10·0% vs. 5·2% of time). Grip strength of women and men was 20·8 (95% CI 20·3–21·3) and 29·4 (95% CI 28·1–30·7) kg. In addition to the shown gender differences, physical activity and capacity differed according to age and education. Participants with little or no education were more active and had higher grip strength than those with secondary school. Likewise, outcomes were higher in younger compared to older participants (data not shown). Consequently, estimates of physical activity and capacity were adjusted for sex, age and education.
Table 2. Physical activity, energy expenditure and physical capacity of HIV patients at initiation of antiretroviral treatment
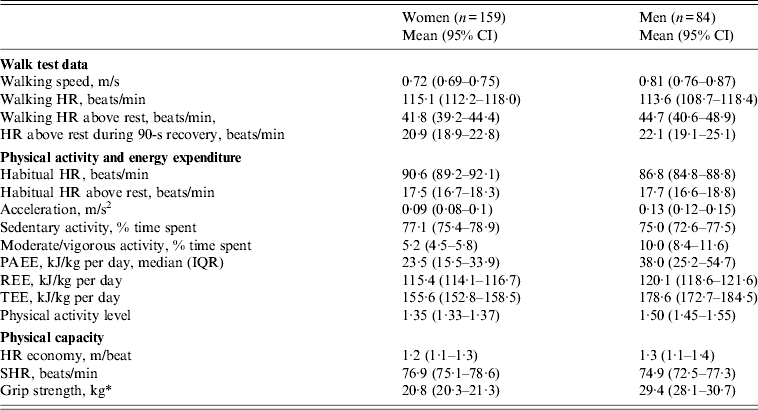
CI, Confidence interval; HR, heart rate; PAEE, physical activity energy expenditure; IQR, interquartile range; REE, resting energy expenditure; TEE, total energy expenditure; SHR, sleeping heart rate.
Data are mean (95% CI) unless otherwise indicated. HR economy is defined as walking distance per HR above rest, m/beat.
* Women (n = 229), men (n = 114).
The associations between habitual physical activity and its predictors are presented in Table 3. Advanced HIV disease was associated with reductions in physical activity as lower CD4, higher viral load, advanced WHO stage and TB co-infection predicted inferior outcomes on all indicators of habitual physical activity. For example, an increase of one unit viral load [log(1+copies/ml)] was associated with −15% PAEE (e B : 0·85 kJ/kg per day, 95% CI 0·80–0·91) and WHO stage IV was associated with −44% PAEE (e B : 0·56 kJ/kg per day, 95% CI 0·44–0·70). Similarly, anaemia was associated with lower activity, e.g. participants with grade 2 anaemia spent 8·9% (95% CI 4·8–12·9) more time in sedentary activity than participants without anaemia. Although anaemia was a strong predictor of physical activity, it did not confound the estimated associations between other predictors and outcomes. Furthermore, low BMI was associated with reduced levels of habitual physical activity. Participants with BMI <16 had −42% PAEE (e B : 0·58, 95% CI 0·46–0·73) and spent 9·0% more time being sedentary compared to BMI >18·5. The associations between BMI and physical activity persisted after adjusting the models for CD4, viral load and WHO stage, respectively, indicating that the impact of being underweight was not merely a mediator of HIV progression (data not shown). An interaction between sex and food security was observed for PAEE (P = 0·03). Food insecurity predicted higher levels of physical activity in men (mild food insecurity, e B : 2·24 kJ/kg per day, 95% CI 1·38–3·64; moderate food insecurity, e B : 1·87 kJ/kg per day, 95% CI 1·20–2·91; severe food insecurity, e B : 1·54 kJ/kg per day, 95% CI 0·98–2·42), while no association was seen in women (P = 0·55). No associations were found between energy intake and physical activity outcomes.
Table 3. Predictors of habitual physical activity of HIV patients at initiation of antiretroviral treatment
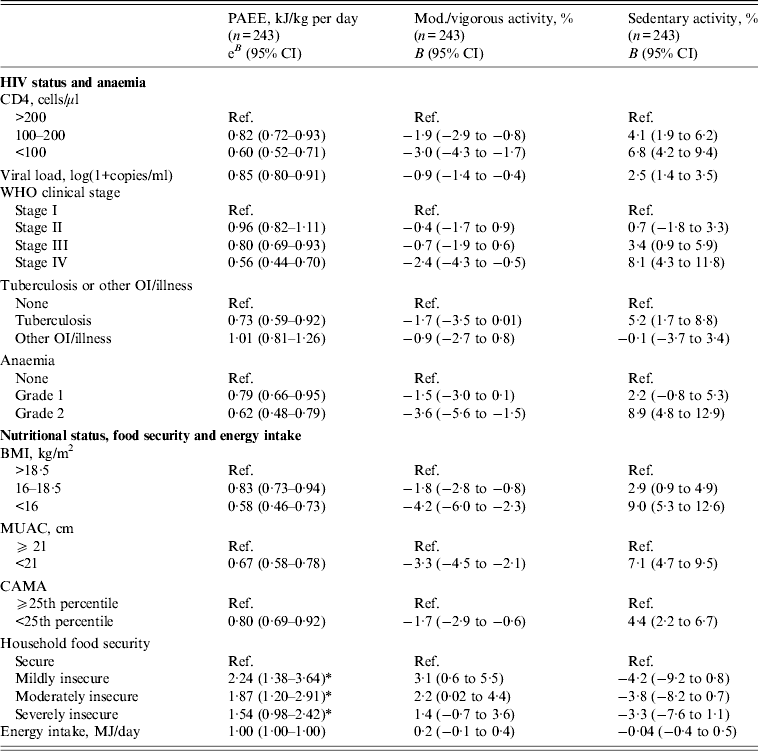
PAEE, Physical activity energy expenditure (effect estimates are back-transformed from log scale); CI, confidence interval; OI, opportunistic infection; BMI, body mass index; MUAC, middle upper arm circumference; CAMA, corrected arm muscle area. Predictors are assessed in individual models adjusted for sex, age and education. PAEE is adjusted for HR economy (walking distance per HR above rest, m/beat). Anaemia grade 1 was defined as haemoglobin (g/l) 105–125 (women) and 117–135 (men). Anaemia grade 2 was defined as haemoglobin (g/l) <105 (women) and <117 (men).
* Estimates refer to men only (cf. Results section for further details).
The associations between physical capacity and its predictors are presented in Table 4. In general, advanced HIV disease was associated with reduced grip strength and increased SHR, but not with HR economy. By adjusting the effect estimates of disease severity on SHR for HR economy, we found that the associations were not confounded by differences in individual fitness (data not shown). Increasing viral load predicted lower grip strength (B: −1·0 kg, 95% CI −1·5 to 0·4), whereas low CD4 did not. An interaction between sex and WHO stage was observed (P < 0·001), showing that advanced WHO stage predicted inferior grip strength in men (WHO stage III: −6·8 kg, 95% CI −10·0 to −3·6; WHO stage IV: −9·0 kg, 95% CI −13·7 to −4·4), but not in women (P = 0·58). Anaemia was associated with lower levels of all indicators of physical capacity, including HR economy, but anaemia was not a confounder of other predictors' associations. Low BMI predicted higher SHR and lower grip strength. BMI <16 was associated with +4·9 beats/min (95% CI 0·3–9·5) SHR and −6·8 kg (95% CI −8·7 to −5·0) grip strength, compared to BMI >18·5. Greater energy intake (MJ) predicted higher grip strength (B: 0·3 kg, 95% CI 0·1–0·5). The estimate was adjusted for weight to ensure the effect was not explained by differences in body size.
Table 4. Predictors of physical capacity of HIV patients at initiation of antiretroviral treatment

HR, Heart rate; SHR, sleeping heart rate; CI, confidence interval; OI, opportunistic infection, BMI, body mass index, MUAC, middle upper arm circumference, CAMA, corrected arm muscle area. Predictors are assessed in individual models adjusted for sex, age and education. Anaemia grade 1 was defined as haemoglobin (g/l) 105–125 (women) and 117–135 (men). Anaemia grade 2 was defined as haemoglobin (g/l) <105 (women) and <117 (men).
* Estimates refer to men only (cf. Results section for further details).
† Adjusted for weight.
Furthermore, grip strength of patients (n = 343) was compared to the HIV-negative reference group (n = 100). The sex- and age distribution of references was similar to that of study participants, while the reference group had higher BMI (+2·3, P < 0·001) and education levels than HIV patients (63% vs. 21% with secondary school or higher, P < 0·001). The comparison showed that grip strength was 4·2 kg lower (95% CI 2·9–5·5) in patients than in an HIV-negative reference group after controlling for age, sex, education and weight (P < 0·001).
Types of activities
The activities reported in recall questionnaires mainly related to job and household activities and showed that more men engaged in job-related activities while more women engaged in household activities. Job activities were reported by 16% of all participants (20% of men and 13% of women). The most common job activity was ‘manual labour at construction sites’, reported by 15% of men and 3% of women. Household activities, such as cleaning, preparing food and washing clothes, were reported by 90% of women and 43% of men. Gender differences were especially related to activities such as washing clothes (28% of women, 10% of men) and carrying water (35% of women, 11% of men). Leisure activities were reported by 7%. These activities included singing, playing music, table tennis and gymnastics. The impact of HIV on activities was evident as 22% of the participants stated the activities of the previous day were influenced by their illness. A further 8% said the previous day did not represent a usual day for them due to holiday or other special occasion.
DISCUSSION
This study showed a strong impact of HIV severity on the functional status of patients at initiation of ART as all indicators of disease status, including CD4, viral load and WHO stage, were strongly associated with low levels of physical activity and capacity. In addition, grip strength was substantially lower in patients than in an HIV-negative reference group. Malnutrition, in terms of low BMI, was identified in half of the patients at initiation of ART and was an independent predictor of low levels of physical activity and capacity.
A previous study among TB patients in Tanzania used similar methods for monitoring physical activity and reported lower activity levels in patients with HIV co-infection [Reference Faurholt-Jepsen7]. However, the present study is the first description of habitual physical activity, physical capacity and their predictors in a large sample of HIV patients in an African setting. The additional previous studies have all been conducted in high-income countries in small patient samples (n = 9–33) [Reference Coors26–Reference Kosmiski30].
The use of objective outcome measurements and long-term assessment of habitual physical activity represent the main strengths of the present study. However, the fact that energy intake and types of physical activity were based on single-day assessments is a limitation, since some day-to-day variation is expected, making it difficult to detect potential associations in the data. The study is further limited by only including a selected population of HIV patients. Since all were eligible for ART at the time of assessment, the variability of HIV severity is reduced. In addition, the cross-sectional study design prevents us from determining causality. Physical activity might be reduced as a consequence of advanced HIV or individuals with higher levels of activity might have better resistance to progression of HIV. An early study suggested that regular exercise might increase CD4 counts [Reference LaPerriere31], but the findings were not reproduced in subsequent trials and meta-analyses have found no effects of physical exercise on virological outcome in HIV [Reference O'Brien32].
The PAEE data that are available from previous studies in HIV patients, have all derived their estimates from activity diaries or calculations based on TEE using doubly-labelled water [Reference Coors26–Reference Kosmiski30]. Most of these studies have reported higher PAEE than we found in the Ethiopian population. Heijligenberg et al. reported a mean±s.d. PAEE of 53 ± 7·8 kJ/kg per day in nine male HIV patients in The Netherlands [Reference Heijligenberg27] and Macallan et al. reported PAEE levels between 32·5 and 88·4 kJ/kg per day in 27 male HIV patients in the UK [Reference Macallan29]. The latter research group described energy intake and expenditure in HIV patients during episodes of weight changes. Only patients undergoing rapid weight loss had PAEE similar to the Ethiopian patients. Macallan et al. found negative energy balances of 34–56 kJ/kg per day in patients undergoing weight loss. In this cross-sectional study, we do not have information on current weight changes but, when comparing TEE with estimated energy intakes, we note a negative energy balance of more than 40 kJ/kg per day, indicating that patients were losing weight at the time of ART initiation. However, reported energy intakes may be somewhat underestimated as its calculation is based on standard recipes of local dishes. The fat content of local dishes might be higher in urban settings and may also have increased, as part of the general nutritional transition in the region [Reference Popkin33], since the food composition tables were updated in 1998 [18].
In addition to the described associations with habitual physical activity, it was found that HIV and nutritional status were also strongly associated with indicators of physical capacity. For example, increasing viral load was associated with increasing SHR. The relevance of SHR as an outcome has been documented in studies linking increased SHR with long-term mortality [Reference Jensen34]. Our findings also support previous reports showing that advanced HIV is associated with reduced grip strength [Reference Jensen34, Reference Raso35]. The use of maximal grip strength has recently been highlighted as a simple, quick and valid measure for assessing physical capacity in HIV studies [Reference Raso35]. Grip strength correlates with mobility [Reference Jakobsen, Rask and Kondrup37] and has been shown to be a strong predictor of morbidity and mortality [Reference Newman38]. Furthermore, reduced grip strength is part of the frailty phenotype (defined as slow walking speed, weak grip strength, low self-reported physical activity, exhaustion, and weight loss [Reference Desquilbet39]) which has been identified as an independent predictor of HIV progression and mortality [Reference Desquilbet40]. While the prevalence of frailty in HIV patients was 3–14% in US settings, a South African study found almost 20% of patients to be affected by frailty [Reference Pathai41]. The higher prevalence is likely to be a result of differences in disease stage, but the physiological impact of HIV may also be greater in a low-income setting where patients are more likely to be malnourished.
This study also describes sex differences in physical activity and capacity of HIV patients. Other studies have found that men are more likely to lose muscle mass during HIV related weight loss, while women are more likely to lose fat mass [Reference Kotler42, Reference Visnegarwala43]. This observation could explain why grip strength was reduced in advanced WHO stages in men, but not women. Furthermore, we saw a sex-specific association between food insecurity and PAEE, which applied to men only. While it is likely that people with compromised food security have higher physical activity as they put more effort into obtaining food, it is not possible from our data to say why this relationship applied to men only.
In conclusion, this study shows that functional outcomes are markedly impaired with progression of disease and malnutrition in Ethiopian HIV patients at initiation of ART. Accurate quantification of physical activity and capacity is a central part of the efforts to understand the impact of HIV on the daily lives of patients and their families, especially in low-income settings where lack of formal social security makes maintenance of work capacity essential. While access to ART has improved greatly in sub-Saharan Africa, the poor nutritional status of patients is often overlooked. Most patients gain weight when started on medical treatment, but physical activity and capacity is not necessarily restored. If patients receive ART without adequate nutritional intake, there is a risk that their weight gain will consist of fat mass only. HIV treatment programmes therefore need to support recovery of these functional outcomes to mitigate the negative effects of the disease.
ACKNOWLEDGEMENTS
The authors thank the participants and study team as well as the staff of the ART clinics at Jimma University Specialized Hospital, Jimma Health Centre and Agaro Health Centre. The study was funded by the US Dairy Export Council. This work was performed at Jimma University Specialized Hospital, Jimma, Ethiopia.
DECLARATION OF INTEREST
None.







