Published online by Cambridge University Press: 17 February 2014
The stability of a suspension of chemotactic bacteria confined in an infinitely long channel and subjected to a stationary, linear chemo-attractant gradient is investigated. While swimming, individual bacteria exert force dipoles on the fluid, which at the continuum level lead to a stress depending upon the bacterial orientation and number density fields. The presence of the attractant gradient causes bacteria to tumble less frequently when swimming along the gradient, leading to a mean orientation and a non-zero chemotactic drift velocity 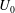 $U_0$ in that direction. At long length and time scales compared to those associated with the persistence of bacterial swimming, fluxes due to chemotaxis and the random run–tumble motion of bacteria balance to yield an exponentially varying number density profile across the channel in the base state. The associated bacterial stress field is also exponentially varying and is normal. This spatially non-uniform base state is unstable to fluctuations in the bacterial concentration field when the scaled bacterial concentration
$U_0$ in that direction. At long length and time scales compared to those associated with the persistence of bacterial swimming, fluxes due to chemotaxis and the random run–tumble motion of bacteria balance to yield an exponentially varying number density profile across the channel in the base state. The associated bacterial stress field is also exponentially varying and is normal. This spatially non-uniform base state is unstable to fluctuations in the bacterial concentration field when the scaled bacterial concentration 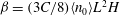 $\beta = (3 C/8) \langle n_0\rangle L^2 H$ exceeds a critical value determined by a Péclet number defined as
$\beta = (3 C/8) \langle n_0\rangle L^2 H$ exceeds a critical value determined by a Péclet number defined as 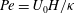 ${\mathit{Pe}} = U_0 H/\kappa $. Here,
${\mathit{Pe}} = U_0 H/\kappa $. Here, 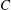 $C$ is a non-dimensional dipole strength, which depends on the geometry of the bacterium,
$C$ is a non-dimensional dipole strength, which depends on the geometry of the bacterium, 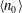 $\langle n_0\rangle $ is the bacterial concentration averaged across the channel of depth
$\langle n_0\rangle $ is the bacterial concentration averaged across the channel of depth  $H$,
$H$, 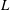 $L$ is the total length of the bacterium,
$L$ is the total length of the bacterium,  $\kappa $ is the bacterial diffusivity, and
$\kappa $ is the bacterial diffusivity, and 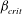 $\beta _{\mathit{crit}}$ is a monotonically decreasing function of
$\beta _{\mathit{crit}}$ is a monotonically decreasing function of 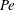 ${\mathit{Pe}}$, with
${\mathit{Pe}}$, with 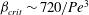 $\beta _{\mathit{crit}} \sim 720/{\mathit{Pe}}^3$ for
$\beta _{\mathit{crit}} \sim 720/{\mathit{Pe}}^3$ for 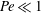 ${\mathit{Pe}} \ll 1$ and
${\mathit{Pe}} \ll 1$ and 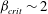 $\beta _{\mathit{crit}} \sim 2$ for
$\beta _{\mathit{crit}} \sim 2$ for 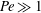 ${\mathit{Pe}} \gg 1$. The instability is the result of the coupling between the active stress-driven fluid flow and the bacterial concentration, and manifests as rectangular convection patterns. When
${\mathit{Pe}} \gg 1$. The instability is the result of the coupling between the active stress-driven fluid flow and the bacterial concentration, and manifests as rectangular convection patterns. When 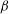 $\beta $ first exceeds
$\beta $ first exceeds 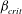 $\beta _{\mathit{crit}}$, the unstable wavelengths are large with
$\beta _{\mathit{crit}}$, the unstable wavelengths are large with 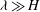 $\lambda \gg H$ and the mode of instability is stationary. Although oscillatory modes appear when
$\lambda \gg H$ and the mode of instability is stationary. Although oscillatory modes appear when  $\lambda \leq O(H)$ and
$\lambda \leq O(H)$ and 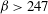 $\beta > 247$, the most dangerous mode of instability is found to be always stationary with a wavelength
$\beta > 247$, the most dangerous mode of instability is found to be always stationary with a wavelength 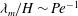 $\lambda _m/H \sim {\mathit{Pe}}^{-1}$. To study the coupling between the previously analysed orientation shear instability mechanism of bacterial suspensions and the new chemotaxis-driven instability, a new set of continuum equations that consistently account for weak chemotaxis, rotation of bacteria by weak fluid shear and weak non-continuum effects along with their coupled effects has been derived. The stability analysis of those equations showed that the orientation shear mechanism has only a negligible influence on the critical concentration for the present chemotaxis-induced instability when the suspension depth is large, and it is the latter that has the lowest critical concentration.
$\lambda _m/H \sim {\mathit{Pe}}^{-1}$. To study the coupling between the previously analysed orientation shear instability mechanism of bacterial suspensions and the new chemotaxis-driven instability, a new set of continuum equations that consistently account for weak chemotaxis, rotation of bacteria by weak fluid shear and weak non-continuum effects along with their coupled effects has been derived. The stability analysis of those equations showed that the orientation shear mechanism has only a negligible influence on the critical concentration for the present chemotaxis-induced instability when the suspension depth is large, and it is the latter that has the lowest critical concentration.