Introduction
The South China Sea (SCS) is a marginal sea that has an estimated area of 3.8 million km2 (Morton & Blackmore, Reference Morton and Blackmore2001), which is bordered by eight countries: China, Taiwan, the Philippines, Malaysia, Brunei, Indonesia, Singapore and Vietnam (McManus et al., Reference McManus, Shao and Lin2010). Despite being the western border of the Coral Triangle, the most biodiverse region in the world, most of the SCS is still relatively unexplored, hence, has received less scientific attention (Gomez, Reference Gomez2015; Huang et al., Reference Huang, Licuanan, Hoeksema, Chen, Ang, Huang, Lnae, Vo, Waheed, Affendi, Yeemin and Chou2015; Juinio-Meñez, Reference Juinio-Menez2015). The few studies that have attempted to quantify the biodiversity of the SCS have indicated that it is comparable to the Coral Triangle in terms of coral diversity, with the highest richness recorded in locations where the SCS overlaps with the Coral Triangle (Huang et al., Reference Huang, Licuanan, Hoeksema, Chen, Ang, Huang, Lnae, Vo, Waheed, Affendi, Yeemin and Chou2015). The SCS is regarded as one of the world's most ecologically significant areas (McManus et al., Reference McManus, Shao and Lin2010) because it is a critical habitat for many endangered animals (McManus, Reference McManus1994), and has the potential to supply larvae to adjacent areas (Dorman et al., Reference Dorman, Castruccio, Curchitser, Kleypas and Powell2015; Juinio-Meñez, Reference Juinio-Menez2015). However, territorial disputes over the SCS and lack of management have resulted in the deterioration of its environment (McManus, Reference McManus1994; Morton & Blackmore, Reference Morton and Blackmore2001), including coral reef ecosystems (Feary et al., Reference Feary, Fowler and Ward2014). Most of the biodiversity surveys conducted in the SCS were on shallow water reefs (SWRs; <30 m depth; Aliño et al., Reference Aliño, Nañola, Ochovillo and Rañola1998; Huang et al., Reference Huang, Licuanan, Hoeksema, Chen, Ang, Huang, Lnae, Vo, Waheed, Affendi, Yeemin and Chou2015), but information on coral reef ecosystems beyond 30 m in the SCS warrant further investigation.
A portion of the eastern SCS overlaps with the Coral Triangle, and is within the jurisdiction of the Philippines (hereby referred to as West Philippine Sea: WPS). The WPS is a distinguished biogeographic area, extending from Batanes, the northernmost group of islands of the Philippines in the Luzon Strait, to the nearshore fringing and offshore atoll reefs of Palawan in the south-western Philippines (Aliño & Gomez, Reference Aliño, Gomez, Yamazato, Ishijima, Sakihara, Taira, Shimabukuro, Teruya and Nishihira1994). Among the six biogeographic regions in the Philippines, the WPS has the highest species richness and biomass of reef fishes (Nañola et al., Reference Nañola, Aliño, Arceo, Licuanan, Uychiaoco, Quibilan, Campos, Alcala, White and Gomez2006, Reference Nañola, Aliño and Carpenter2010). For scleractinian corals, there are no published reports comparing coral richness among biogeographic regions; but for percentage cover, the WPS is not particularly exceptional (Gomez et al., Reference Gomez, Aliño, Yap and Licuanan1994; Magdaong et al., Reference Magdaong, Fujii, Yamano, Licuanan, Maypa, Campos, Alcala, White, Apistar and Martinez2014). The average coral cover in the WPS is 25%, which is barely above the average coral cover (22%) of the Philippines (Licuanan et al., Reference Licuanan, Robles, Dygico, Songco and van Woesik2017). Because of the large area covered by the WPS, there are within-WPS spatial differences in coral species richness (Huang et al., Reference Huang, Licuanan, Hoeksema, Chen, Ang, Huang, Lnae, Vo, Waheed, Affendi, Yeemin and Chou2015), coral cover (Gomez et al., Reference Gomez, Aliño, Yap and Licuanan1994; Licuanan et al., Reference Licuanan, Robles, Dygico, Songco and van Woesik2017), fish species richness (Nañola et al., Reference Nañola, Aliño and Carpenter2010) and biomass (Nañola et al., Reference Nañola, Aliño, Arceo, Licuanan, Uychiaoco, Quibilan, Campos, Alcala, White and Gomez2006). However, similar to studies in the SCS, biodiversity surveys in the WPS were limited to SWRs, excluding relatively deep ecosystems, such as mesophotic coral ecosystems (MCEs).
Mesophotic coral ecosystems (MCEs) are situated at a depth range of 30–150 m depth, and are distinguished by their relatively lower light levels from SWRs, i.e. 1–10% lower (Lesser et al., Reference Lesser, Slattery and Leichter2009). MCEs can be further categorized according to depth, with ‘upper’ (30–59 m), ‘middle’ (60–89 m) and ‘lower’ mesophotic (90–150 m) divisions (Pinheiro et al., Reference Pinheiro, Goodbody-Gringley, Jessup, Shepherd, Chequer and Rocha2016; Rocha et al., Reference Rocha, Pinheiro, Shepard, Papastamatiou, Luiz, Pyle and Bongaerts2018). The growing interest in MCEs is attributed to the potential refugia that these ecosystems provide (Bongaerts et al., Reference Bongaerts, Ridgway, Sampayo and Hoegh-Guldberg2010; Bridge et al., Reference Bridge, Done, Friedman, Beaman, Williams, Pizarro and Webster2011; Lindfield et al., Reference Lindfield, Harvey, Halford and McIlwain2016), together with their inferred capabilities to reseed degraded SWRs (Bongaerts et al., Reference Bongaerts, Ridgway, Sampayo and Hoegh-Guldberg2010, Reference Bongaerts, Riginos, Brunner, Englebert, Smith and Heogh-Guldberg2017); though other reports suggest that MCEs are just as vulnerable as SWRs (e.g. Quimpo et al., Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018b; Rocha et al., Reference Rocha, Pinheiro, Shepard, Papastamatiou, Luiz, Pyle and Bongaerts2018), hence should be studied to appropriately craft management interventions. Surveys of MCEs across the globe have shown that depth structures ecological communities by shaping distinct assemblages along a depth gradient (Semmler et al., Reference Semmler, Hoot and Reaka2016; Baldwin et al., Reference Baldwin, Tornabene and Robertson2018; Rocha et al., Reference Rocha, Pinheiro, Shepard, Papastamatiou, Luiz, Pyle and Bongaerts2018), which is characterized by the decrease in biodiversity and abundance (Lesser et al., Reference Lesser, Slattery and Leichter2009; Kahng et al., Reference Kahng, Garcia-Sais, Spalding, Brokovich, Wagner, Weil, Hinderstein and Toonen2010; Semmler et al., Reference Semmler, Hoot and Reaka2016; Rocha et al., Reference Rocha, Pinheiro, Shepard, Papastamatiou, Luiz, Pyle and Bongaerts2018), together with changes in morphological features (e.g. corals; Soto et al., Reference Soto, De Palmas, Ho, Denis and Chen2018) and increasing size for certain taxa in some locations (e.g. fishes; Lindfield et al., Reference Lindfield, Harvey, Halford and McIlwain2016; Quimpo et al., Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018b). Despite the differences however, assemblage overlap may also occur, particularly at the upper mesophotic zone (Slattery et al., Reference Slattery, Lesser, Brazeau, Stokes and Leichter2011) due to hydrological processes that advect the early planktonic stages of reef animals (Holstein et al., Reference Holstein, Paris, Vaz and Smith2015), together with the high motility of some fauna (e.g. fishes; Tenggardjaja et al., Reference Tenggardjaja, Bowen and Bernard2014; Papastiamatou et al., Reference Papastiamatou, Meyer, Kosaki, Wallsgrove and Popp2015).
Although substantial work has been conducted in many parts of the world (reviewed in Turner et al., Reference Turner, Babcock, Hovey and Kendrick2017), MCEs in the biodiverse Indo-Pacific are still under-studied (Kahng et al., Reference Kahng, Garcia-Sais, Spalding, Brokovich, Wagner, Weil, Hinderstein and Toonen2010). Currently, there are only a handful of MCE studies in the Philippines (Ross & Hodgson, Reference Ross and Hodgson1981; Abesamis et al., Reference Abesamis, Langlois, Birt, Thillainath, Bucol, Arceo and Russ2017; Nacorda et al., Reference Nacorda, Dizon, Meñez, Nañola, Roa-Chio, De Jesus, Hernandez, Quimpo, Licuanan, Aliño and Villanoy2017; Quimpo et al., Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018a, Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringanb; Cabaitan et al., Reference Cabaitan, Quimpo, Dumalagan, Munar, Calleja, Olavides, Go, Albelda, Cabactulan, Tinacba, Doctor, Villanoy, Siringan, Loya, Puglise and Bridge2019), the centre of marine biodiversity (Carpenter & Springer, Reference Carpenter and Springer2005; Veron et al., Reference Veron, Devantier, Turak, Green, Kininmonth, Stafford-Smith and Peterson2009), with only three studies conducted in a few locations in the WPS biogeographic region (Ross & Hodgson, Reference Ross and Hodgson1981; Quimpo et al., Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018a, Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringanb), though none have focused specifically on comparing multiple locations within the WPS.
In this study, we examined the benthic habitat, coral genera and fish species assemblages in the upper mesophotic (30–40 m) and SWRs at three locations (Kalayaan Group of Islands, Palawan and Luzon Strait) in the eastern South China Sea, locally known as the West Philippine Sea, using diver-based survey methods. Specifically, this study examined the differences in benthic habitat (i.e. benthic percentage cover), coral genera (i.e. generic richness and abundance) and fish species (i.e. fish species richness, abundance and biomass) assemblages among locations and between depths (SWRs and upper MCE) in the WPS. We hypothesize that (1) there are differences in benthic habitat, coral and fish assemblages among locations, and (2) there are differences between SWRs and MCEs. This study will improve our understanding of biodiversity of MCEs in a portion of the South China Sea, particularly in the West Philippine Sea.
Materials and methods
Study locations
The study locations were in the Kalayaan Group of Islands (hereafter referred to as KIG), which is also referred to as the Spratly Group of Islands; Quezon municipality in Palawan (Pal); and the Luzon Strait (LS), specifically in the islands of Batanes located in the eastern South China Sea (Figure 1). The KIG is an offshore reef system composed of numerous islands and islets that are located ~520 km to the west of Palawan mainland, with the last survey conducted 20 years ago, due to logistical constraints (Aliño et al., Reference Aliño, Nañola, Ochovillo and Rañola1998). In the present study, we surveyed four islands at the KIG: Pag-asa, Lawak, Northeast Investigator and Sabina. Pal is a nearshore reef system located in western Palawan, and is regarded as one of the Philippines' last ecological frontiers, though some sites are still heavily exploited by the Live Reef Fish Food Trade (LRRFT; Fabinyi & Dalabajan, Reference Fabinyi and Dalabajan2011; Fabinyi et al., Reference Fabinyi, Pido, Harani, Caceres, Uyami-Bitara, De las alas, Buenconsejo and Ponce de Leon2012). LS is a nearshore island reef system that is located in the northernmost tip of the Philippines, connecting the SCS with the Pacific Ocean. The three study locations are exposed to comparable mean sea surface temperature (~31.40 °C) at minimum depth; however, LS is exposed to stronger current velocity at 1.138 m−1 compared with Pal (0.063 m−1) and KIG (0.032 m−1) (Assis et al., Reference Assis, Tyberghein, Bosh, Verbruggen, Serrão and De Clerck2017). As for anthropogenic disturbances and governance, all of the locations experience medium to very high levels of integrated threats on a localized scale (Burke et al., Reference Burke, Reytar, Spalding and Perry2012), and all of the locations surveyed were not within marine protected areas (MPAs).
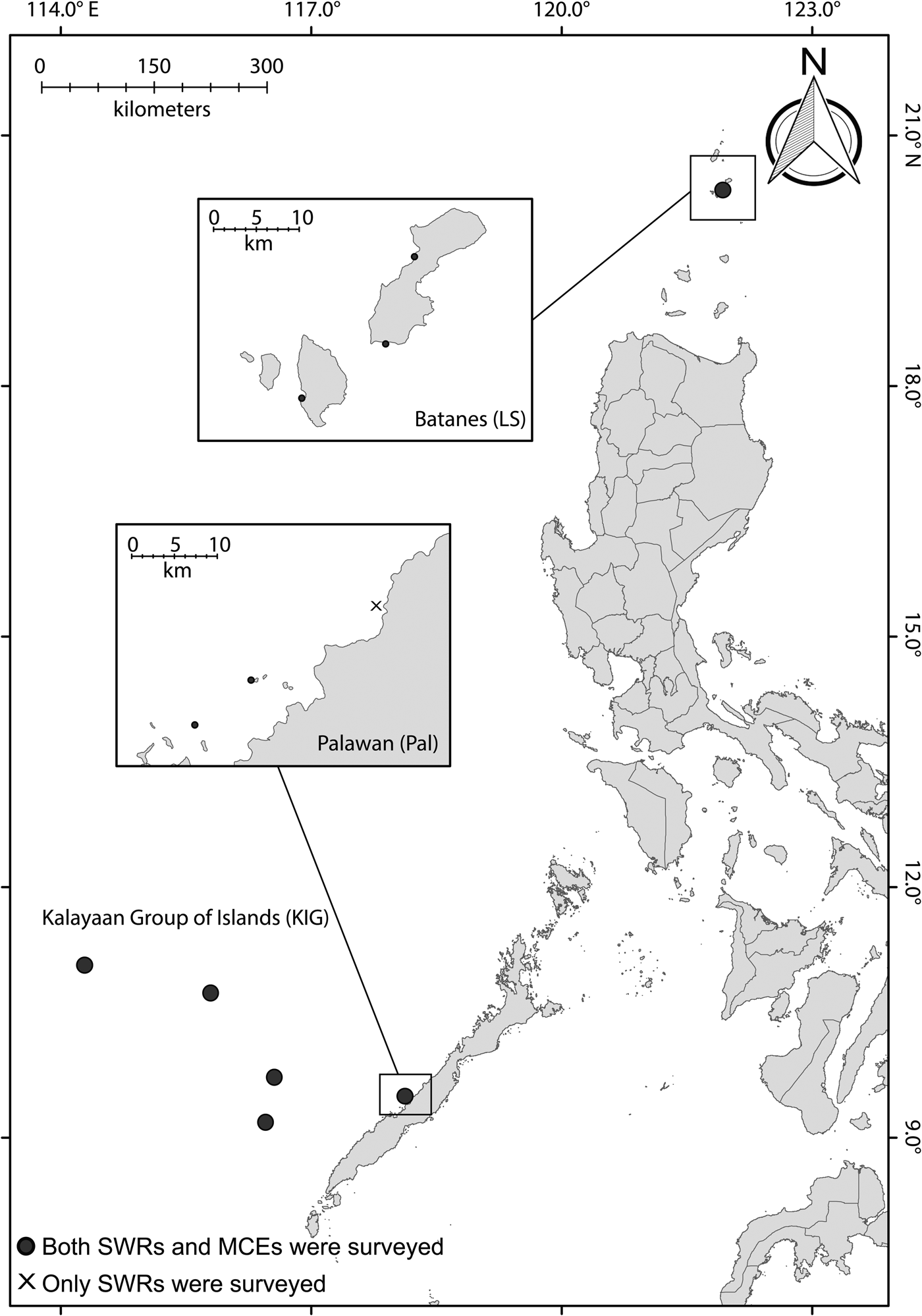
Fig. 1. Map showing the three study locations (KIG, Pal and LS) in the Western Philippine Sea.
Sampling
The photo-quadrat method was used to assess the benthic and coral assemblages at the three locations. Each location had different numbers of sampling sites and depths (KIG: 4 SWRs and 4 MCEs; Pal: 3, 2; and LS: 3, 3), with SWR depths that ranged from 8 to 22 m, while upper MCE depths ranged from 30–40 m. In all sites, the MCEs were contiguous with SWRs, with the reef morphology either sloping or vertical. Logistical constraints and safety reasons allowed us to survey only one 25 m transect per sampling site, with a survey time of 12–15 min in the upper mesophotic, hence within-location differences in benthic and fish assemblage structures could not be examined. Photographs of the benthos were taken every 1 m interval along the transect using a Canon G1-X underwater camera, with the images analysed using Coral Point Count with Excel extensions (CPCe; Kohler & Gill, Reference Kohler and Gill2006). In each image, 25 sampling points (5 × 5 uniform grid) were overlaid, and the benthic organisms that were intercepted by the points were identified as hard coral, dead coral or bare hard substrate, soft coral, algae assemblages (e.g. turf and macroalgae) and invertebrates. Abiotic components, such as sand and silt were also recorded. Because of the relatively short survey time in the present study, corals could not be identified down to species levels, but instead were identified to genera level, which provides a good estimate of diversity given logistical and time constraints as demonstrated by surveys in Singapore (Dikou & van Woesik, Reference Dikou and van Woesik2006) and Australia (Bridge et al., Reference Bridge, Done, Friedman, Beaman, Williams, Pizarro and Webster2011). Coral genera richness, morphology and abundance were obtained from the benthic images, wherein every coral colony observed was identified down to genus level by a single assessor for consistency (always E. Dumalagan) along with their respective morphological characteristics (i.e. tabulate, branching, encrusting, foliose, massive, solitary and submassive) and abundance.
To assess the fish species assemblages, a modified fish visual census (FVC) method was used, wherein we surveyed a 25 m transect length, but with a width of 10 m (i.e. 5 m belt to the left and 5 m belt to the right of the transect), instead of the suggested 5 m (2.5 m per side) in English et al. (Reference English, Wilkinson and Baker1997). We extended the FVC width to compensate for the short transect length. Appropriate standardization was conducted by divers (TJ Quimpo and KT Go) prior to the actual field surveys to ensure accurate species identification, count and size estimate even if fish are situated 5 m away from the transect. During the actual surveys, divers recorded all fish encountered, identifying them down to species level, counting their abundance and estimating their length (total length in cm). Biomass of each individual fish was obtained using the formula W = aLb where a and b are species-specific growth coefficients per fish species, and L is the estimated length (Froese & Pauly, Reference Froese and Pauly2018). Trophic groups per fish species were determined based on their dominant diet gleaned from the online repository www.fishbase.org (Froese & Pauly, Reference Froese and Pauly2018).
Statistical analyses
PERMANOVA (Anderson, Reference Anderson2001, Reference Anderson2017; Anderson & Walsh, Reference Anderson and Walsh2013), with each species as a response variable, was used to discern differences in coral and fish abundance, together with fish biomass among locations (three levels: KIG, Pal and LS) and between depths (SWR and MCE) using Bray–Curtis dissimilarity coefficient. To visually inspect the differences in the multidimensional cloud of data for coral genera (abundance-weighted) and fish species (abundance- and biomass-weighted) assemblages, we used constrained analysis of proximities (CAP) on square-root transformed abundance and biomass. The constraints on the coral and fish assemblages were location, depth, benthic characteristics (i.e. hard coral, bare hard substrate, soft coral, invertebrates, algae assemblages and abiotic cover) and coral morphology (i.e. branching, encrusting, foliose, massive, mushroom and submassive abundance). Coral genera or fish species, together with benthic characteristics and coral morphologies that contributed significantly (P < 0.01) to the CAP results were identified by correlating genera/species, benthic characteristic and coral morphology scores to the CAP axes. This analysis produces arrows wherein the direction indicates the most rapid change in the genera/species, benthic characteristic and coral morphology, while the length of the arrow depicts the strength (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2017).
Differences in coral genera and fish species richness among locations and between depths were investigated using a permutation test by Rossi (Reference Rossi2011). The test computes for the difference in the number of genera/species between community 1 and 2 (referred to as d), and then compares this value to n differences in d random obtained from permutating the samples between communities. All the permutational analyses employed 5000 permutations.
All statistical analyses were implemented in R statistical software (R Core Team, 2018), where the multivariate analyses were conducted using the package vegan (Oksanen et al., Reference Oksanen, Blanchet, Friendly, Kindt, Legendre, McGlinn, Minchin, O'Hara, Simpson, Solymos, Stevens, Szoecs and Wagner2017). The genera/species richness calculations and comparisons were implemented in the package rich (Rossi, Reference Rossi2011).
Results
Overall, bare hard substrate had the highest percentage cover among the three locations and between depths, covering 30–65% of the benthos (Figure 2). Coral cover ranged from 7–38%, with Pal having the highest mean coral cover (34 ± 3.18 SE) followed by KIG (20 ± 2.00) and LS (8 ± 2.03). In Pal and KIG, coral cover was slightly higher in SWRs (Pal: 38%; KIG: 22%) compared with MCEs (Pal: 30%; KIG: 18%), while the opposite was recorded in LS (SWR: 7%; MCE: 9%). Coral generic richness was higher but not statistically significant in Pal, followed by KIG and LS. Richness between depths was location-specific, with coral generic richness higher in SWRs (28.16 ± 1.09 SE genus 25 m−2) than MCEs (23.00 ± 0.00) in Pal; while the opposite was recorded in LS (SWR: 13.33 ± 1.66; MCE: 17.66 ± 2.33) and KIG (22.25 ± 1.93; 25.25 ± 2.59) (Figure 3A). However, differences in richness between depths were also not statistically significant. Between the two depths, 19–30% of the genera were shared, with Pal (30%) having the highest percentage of genera shared, followed by KIG (27%) and LS (19%). Mean coral abundance was higher in SWRs than MCEs in KIG (16.95 ± 2.74 SE individuals 25 m−2 and 9.55 ± 1.52 SE individuals 25 m−2, respectively), while no noticeable difference was recorded in LS and Pal (Figure 3B). The dominant morphologies of the corals were both location- and depth-specific. In the SWRs of LS, the submassive morphology was the most dominant, while encrusting, massive and foliose dominated Pal and KIG (Figure 3C). In MCEs for all locations, encrusting was the most dominant morphology (Figure 3D), with foliose also abundant in Pal.

Fig. 2. Percentage contribution of the different benthic categories among locations and between depths.
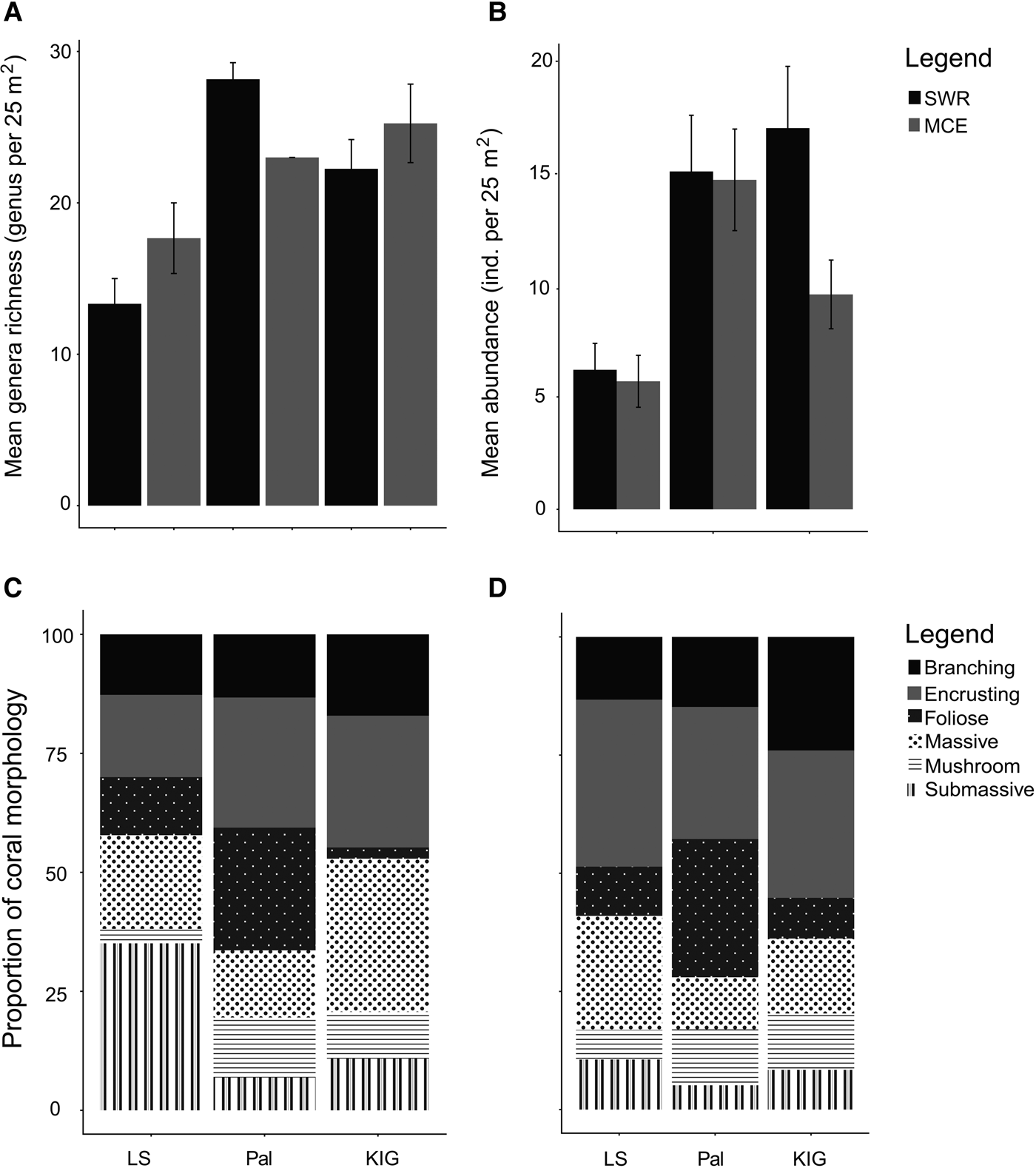
Fig. 3. Coral genera assemblages among locations and between depths. (A) and (B) show mean coral genera richness and mean abundance (individuals per 25 m2). (C) and (D) show the proportional coral morphology in SWRs and MCEs, respectively.
PERMANOVA results suggested that location was the main contributor to the differences in coral genera assemblages (Table 1). This finding was supported by the CAP results wherein sampling sites from the same locations clustered together regardless of depth (Figure 4). Fitted vectors indicated that abiotic cover influenced the coral assemblage in LS, while bare hard substrate and the genera Porites influenced the assemblage in KIG. In Pal, hard coral cover, the encrusting and foliose morphology, and six genera (Oxypora, Pachyseris, Alveopora, Mycedium, Euphyllia and Merulina) influenced the coral assemblages.

Fig. 4. CAP results for coral genera (abundance-weighted) assemblages among locations and between depths. Benthic composition, coral genera and morphology that contributed significantly (P < 0.01) to the variation are displayed as vectors, with the direction indicating the most rapid change and length the strength.
Table 1. PERMANOVA results of coral genera (abundance-weighted) and fish species (abundance- and biomass-weighted) assemblages in response to location, depth and the interaction between the two variables.

Results are based on Bray–Curtis dissimilarity coefficient with 5000 permutations with P values. Significant values are in bold.
Fish species richness decreased with depth in all locations, with LS having the highest mean richness decline (SWR: 25 ± 4.93 SE species 250 m−2; MCE: 17 ± 2.88 SE species 250 m−2), followed by KIG (21 ± 2.98; 15 ± 2.65) and Pal (16 ± 3.17; 11 ± 0.00) (Figure 5A). Shared species between depths ranged from 13–22%, with LS having the greatest proportion of shared species (22%), followed by KIG (19%) and Pal (13%). Fish abundance also decreased, albeit slightly with depth in all locations: LS (SWR: 25.11 ± 5.64 SE individuals 250 m−2; MCE: 22.43 ± 6.89 SE individuals 250 m−2), Pal (16.35 ± 8.69; 14.42 ± 5.87) and KIG (21.88 ± 4.81; 20.50 ± 7.44) (Figure 5B). Fish biomass decreased with depth for LS (SWR: 0.72 ± 0.35 SE kg 250 m−2; MCE: 0.56 ± 0.26 SE kg 250 m−2), while there was no noticeable difference in Pal (0.15 ± 0.03; 0.12 ± 0.04) and KIG (1.16 ± 0.54; 1.13 ± 0.50) (Figure 5C). In all locations, the most abundant trophic group for SWRs was herbivores; while planktivores dominated the MCEs of LS and KIG (Figure 6A, B) In the MCE of Pal, herbivorous fishes had the highest abundance (Figure 6B). The trophic groups that contributed to biomass were location- and depth-specific. Benthic invertivores, herbivores and piscivores had the highest biomass in LS, Pal and KIG, respectively for SWRs (Figure 6C). In MCEs, omnivores had the highest biomass in LS, benthic invertivores in Pal and piscivores in KIG (Figure 6D).
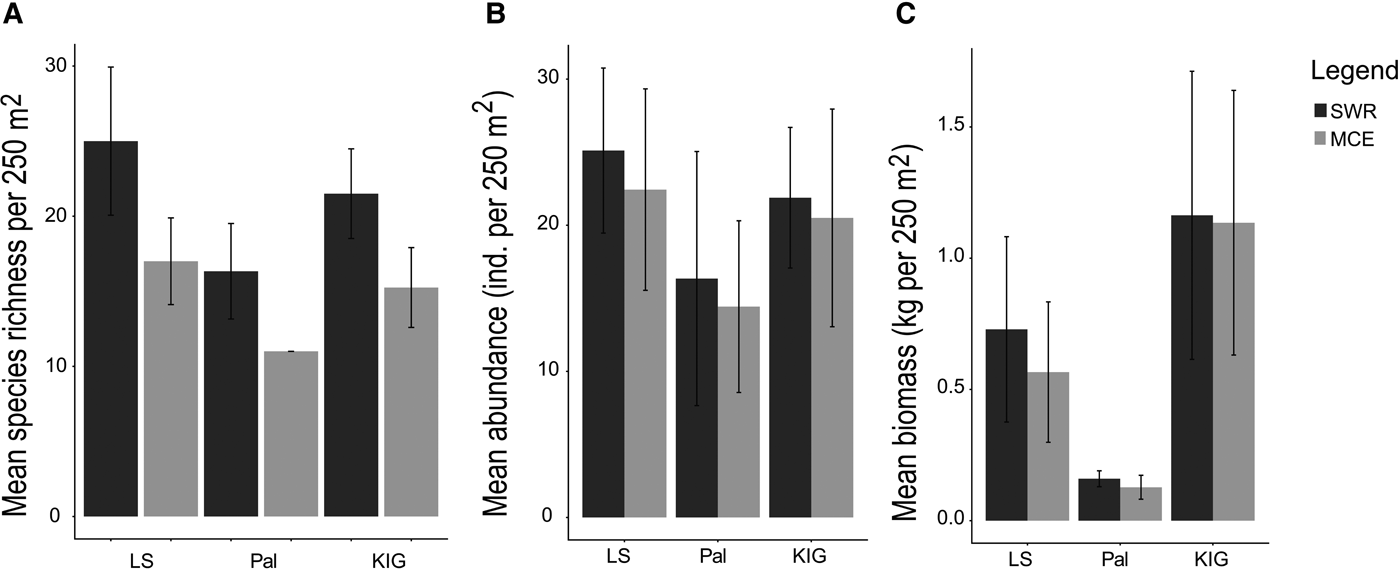
Fig. 5. Fish species assemblages among locations and between depths. (A) and (B) show mean fish species richness and abundance (individuals per 250 m2). (C) shows mean fish biomass in kg per 250 m2.

Fig. 6. Percentage contribution of fish trophic groups to fish abundance and biomass among locations and between depths. (A) and (C) are abundance and biomass for SWRs, while (B) and (D) are for MCEs.
PERMANOVA results for fish species assemblages (abundance- and biomass-weighted) also showed that location had a greater contribution to the dissimilarity than depth (Table 1). Indeed, the CAP results (Figure 7A, B) indicated that sampling sites from the same location clustered together. However, some sites from LS and KIG were situated close to one another in the multidimensional space. Fitted vectors suggested that for abundance-weighted fish species assemblages, LS was mostly associated with abiotic cover, while the branching coral morphology, together with four fish species (Lutjanus kasmira, Dascyllus reticulatus, D. trimaculatus and Plectroglyphidodon lacrymatus) were predominantly associated with KIG. In Pal, hard coral cover, mushroom, foliose and encrusting coral morphologies, along with five fish species (Neoglyphidodon nigroris, Chrysiptera rollandi, Cheilinus fasciatus, Pomacentrus stigma and Bodianus mesothorax) were associated with the abundance-weighted fish species assemblages. Biomass-weighted fish species assemblages for LS and for some sites in KIG were associated with Acanthurus pyroferus. Other sites in KIG were associated with soft corals and Carangoides orthogrammus. In Pal, hard coral cover, foliose coral morphology and four fish species (Cheilodipterus macrodon, Chysiptera rollandi, Plectroglyphidodon nigromarginatus and Pomacentrus stigma) were associated with the biomass-weighted fish species assemblages.
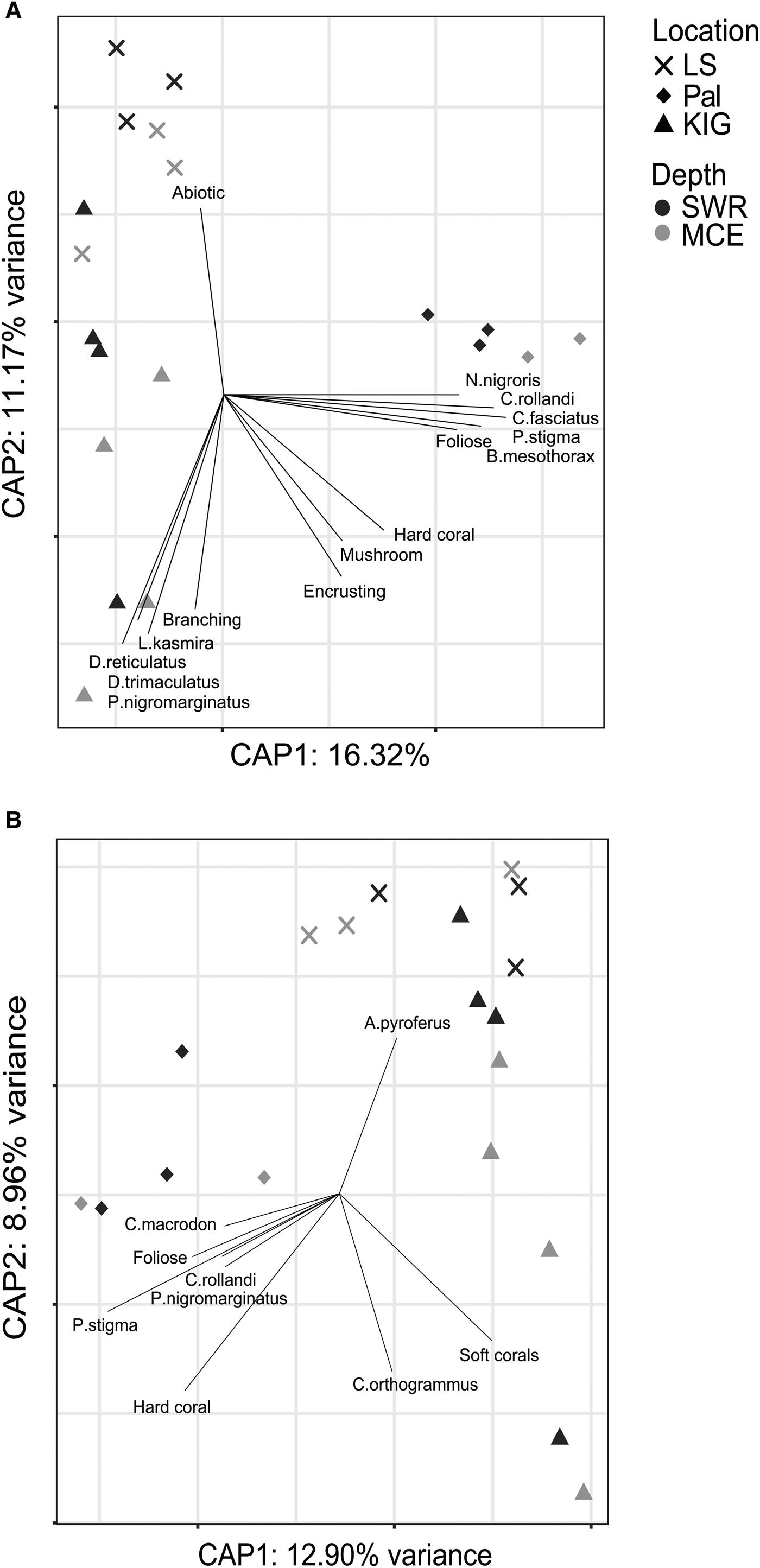
Fig. 7. CAP results for fish species (A. abundance- and B. biomass-weighted) assemblages among locations and between depths. Benthic composition, fish species and coral morphology that contributed significantly (P < 0.01) to the variation are displayed as vectors, with the direction indicating the most rapid change and length indicating the strength.
Discussion
Information on mesophotic coral ecosystems (MCEs) is lacking in the South China Sea (SCS; Randall & Lim, Reference Randall and Lim2000; Huang et al., Reference Huang, Licuanan, Hoeksema, Chen, Ang, Huang, Lnae, Vo, Waheed, Affendi, Yeemin and Chou2015) despite being of considerable biological importance. Here, we recorded 17–25 mean coral genera (genus 25 m−2) and 11–17 mean fish species (species 250 m−2) in the upper MCEs of the eastern SCS, locally known as the West Philippine Sea (WPS). However, when the coral genera and fish species recorded in the present study were compared with species checklists that span the SWRs of the SCS (Randall & Lim, Reference Randall and Lim2000; Licuanan, Reference Licuanan2009; Huang et al., Reference Huang, Licuanan, Hoeksema, Chen, Ang, Huang, Lnae, Vo, Waheed, Affendi, Yeemin and Chou2015), none of the corals and fishes recorded were novel, but we showed that the depth distributions of these genera/species could reach the upper MCEs (Supplementary Material 1). Coral genera and fish species assemblages varied across the three geographic locations for both shallow water reefs (SWR) and upper mesophotic ecosystems (MCEs), and to a lesser extent between depths. Location differences were probably associated with differences in benthic cover, fishing pressure, inherent environmental conditions (e.g. current velocity) and oceanographic features. The lower variation of coral and fish assemblages between depths were probably also associated with benthic cover, interconnectivity between SWRs and MCEs due to hydrological conditions, and vagility of fishes. Indeed, a proportion of genera/species were shared between SWR and MCEs for coral genera (19–30%) and fish species (13–22%).
Differences in coral genera assemblages among locations were associated with the differences in benthic cover. Coral richness and abundance was lowest in LS, the location that was characterized by high abiotic cover, while locations characterized by hard coral cover and bare hard substrate had higher richness and abundance. Abiotic cover (particularly sand and silt) can likely limit the number of genera and individuals that successfully recruit into reefs because of sediment burial (Rogers, Reference Rogers1990; Jones et al., Reference Jones, Ricardo and Negri2015). Coral recruitment, and subsequent growth require the presence of ‘free space’ or bare hard substrate (Gomez et al., Reference Gomez, Cabaitan, Yap and Dizon2014), provided it has been colonized with settlement inducing crustose coralline algae (Heyward & Negri, Reference Heyward and Negri1999) and microbial biofilm (Tran & Hadfield, Reference Tran and Hadfield2011). The higher cover of bare hard substrate in Pal and KIG may have increased the suitable substrata for corals to recruit onto; while the higher hard coral cover and abundance in the two locations, probably increased the reproductive output that improved recruitment (Chiappone & Sullivan, Reference Chiappone and Sullivan1996; Connell et al., Reference Connell1997). Indeed in the present study, the location (Pal) with the highest hard coral cover was characterized by the high abundances of six coral genera (Oxypora, Pachyseris, Alveopora, Mycedium, Euphyllia and Merulina).
The differences in fish species assemblages among locations were also likely influenced by the benthic cover, and to a lesser extent the local fishing techniques. Interestingly, fish species richness and abundance were slightly higher in LS compared with the other two locations despite having lower hard coral cover. Hard coral cover is known to positively improve fish richness and abundance (Bell & Galzin, Reference Bell and Galzin1984; Gratwicke & Speight, Reference Gratwicke and Speight2005; Harborne et al., Reference Harborne, Mumby and Ferrari2013; Komyakova et al., Reference Komyakova, Munday and Jones2013), hence the higher richness and abundance in the structurally less complex habitats in LS was surprising. Trophic structure in the LS however, has revealed that herbivores and benthic invertivores were abundant groups, probably because of the high algae (17%) and abiotic cover (35%) in LS as algae is a vital component of the former's diet, while sand is the latter's foraging habitat. Indeed, previous studies have suggested that not all fish families respond positively to hard coral; instead some fish families (e.g. Acanthuridae, Labridae: Scarinae, and Mullidae) respond positively to low hard coral cover (Shibuno et al., Reference Shibuno, Hashimoto, Abe and Takada1999; Russ et al., Reference Russ, Miller, Rizzari and Alcala2015a, Reference Russ, Questel, Rizzari and Alcala2015b). Fish biomass was lower in Pal and LS relative to KIG, probably because these locations were nearshore, thus were likely more accessible to fishing. Pal is a known location for the Live Reef Fish Food Trade (LRFFT), which began as early as the 1990s (Fabinyi & Dalabajan, Reference Fabinyi and Dalabajan2011), with most of the catch exported as luxury seafood to China (Fabinyi et al., Reference Fabinyi, Pido, Harani, Caceres, Uyami-Bitara, De las alas, Buenconsejo and Ponce de Leon2012). Fishing in LS on the other hand is not as pervasive, since fishing is usually for local consumption with most targeted fishes being pelagic (e.g. dorado or dolphinfish) (Mangahas, Reference Mangahas2010). The reason for the low biomass in LS is unclear, but might be related to the low abundance of large-bodied piscivores, and the low coral cover as some large predatory fishes also rely on hard corals (Kerry & Bellwood, Reference Kerry and Bellwood2012; Khan et al., Reference Khan, Goatley, Brandl, Tebbett and Bellwood2017). The higher biomass in KIG was probably associated with the large sizes of the piscivore Carangoides orthogrammus that may have been inaccessible to most fishers due to the offshore nature of this location (Sampson, Reference Sampson1991; Friedlander & DeMartini, Reference Friedlander and DeMartini2002). However, some commercial fishing vessels can access the KIG, with reports of declining catch for certain fishes, particularly top-level predators (e.g. sharks) (Aliño et al., Reference Aliño, Nañola, Ochovillo and Rañola1998).
Together with benthic cover and local fishing techniques, the inherent environmental conditions and oceanographic features of each location may also have influenced the dissimilarity in coral and fish assemblages. Water motion (represented by current velocity in the present study; Assis et al., Reference Assis, Tyberghein, Bosh, Verbruggen, Serrão and De Clerck2017) was reported to vary among locations, and may have also influenced the dissimilarity in coral and fish assemblages. Considerable changes in species composition for both coral and fish are observed along a water motion gradient, with massive or submassive corals, and fishes with tapered or elongate fins relatively dominant in areas of strong water motion (Done, Reference Done1982; Fulton & Bellwood, Reference Fulton and Bellwood2004). Indeed in the SWRs of LS (the location with the highest water motion), the percentage contribution of the submassive morphology was greater than Pal and KIG. Evaluating differences in fin shape of fishes in the present study is however difficult as appropriate geometric shape analysis is necessary; but previous studies have shown that even within small distances (~100 km), there are intra-specific differences in fin shape associated with water motion (Cabasan et al., Reference Cabasan, Solier, Manual, Paradela and Nañola2017). Aside from environmental conditions, the oceanographic features of each location may have shaped the dissimilarity among locations. The circulation pattern in the WPS is that waters from the Pacific enter via the LS, moving to the island of Palawan, or to the Sulu Sea via the Mindoro Strait (Hulburt et al., Reference Hulburt, Metzger, Sprintall, Riedlinger, Arnone, Shinoda and Xu2011). However, aside from receiving waters from the Pacific, waters also enter the WPS via the western SCS (Kool et al., Reference Kool, Paris, Barber and Cowen2011). The greater water sources (and associated waterborne particles such as larvae) of Palawan (where Pal and KIG are located) may explain why coral diversity was higher than LS as the number of initial species colonizers was larger. The higher fish diversity of LS than KIG and Pal however, could not be explained by the number of water sources, but may be due to local-scale factors (e.g. fishing).
The lower differences in coral genera and fish species assemblages between depths were probably caused by the similarities in benthic composition, interconnectivity between SWRs and MCEs, and fishing efforts. Perhaps the most likely reason for this low variation is the similar benthic composition between depths, particularly the similar percentage cover of bare hard substrate and hard corals, together with coral abundance in SWRs and MCEs that provided adequate substrate for settlement, and likely improved reproductive output, respectively (Chiappone & Sullivan, Reference Chiappone and Sullivan1996; Connell, Reference Connell1997; Hughes et al., Reference Hughes, Graham, Jackson, Mumby and Steneck2010; Gomez et al., Reference Gomez, Cabaitan, Yap and Dizon2014). There were slight changes in coral morphology with depth, with most corals in MCEs displaying either an encrusting or foliose morphology. The abundance of these coral morphologies in MCEs is likely to improve light absorption as photosynthetic active radiation is lower relative to that available for SWRs (Lesser et al., Reference Lesser, Slattery and Leichter2009; Kahng et al., Reference Kahng, Garcia-Sais, Spalding, Brokovich, Wagner, Weil, Hinderstein and Toonen2010). Similarly, fishes are also reliant on hard coral cover for habitat, with higher diversity and abundance recorded in sites with high coral cover (Bell & Galzin, Reference Bell and Galzin1984; Harborne et al., Reference Harborne, Mumby and Ferrari2013; Komyakova et al., Reference Komyakova, Munday and Jones2013).
Alternatively, interconnectivity between depths may also have contributed to the low differences in coral genera and fish species assemblages. For coral assemblages, connectivity can only occur via the transport of their early life stages (i.e. larvae) through vertical advection (Holstein et al., Reference Holstein, Paris, Vaz and Smith2015). A likely mechanism for this vertical advection is through upwelling that has been documented to occur in the SCS region (Kuo et al., Reference Kuo, Zheng and Ho2000; Jing et al., Reference Jing, Qi, Hua and Zhang2008), with upward water motion documented from a depth of 300 m (Shaw et al., Reference Shaw, Liu, Pai and Liu1996). Indeed in the present study, 19–30% of coral genera were shared between SWRs and MCEs. For motile fauna such as fishes, interconnectivity can also occur via the movement of individuals (Slattery et al., Reference Slattery, Lesser, Brazeau, Stokes and Leichter2011), traversing numerous depth strata to forage for food (Papastiamatou et al., Reference Papastiamatou, Meyer, Kosaki, Wallsgrove and Popp2015; Pinheiro et al., Reference Pinheiro, Goodbody-Gringley, Jessup, Shepherd, Chequer and Rocha2016). In the present study, 13–22% of fish species were shared between SWRs and MCEs, with slightly similar abundance for planktivores. Fishing efforts may also have contributed to the similarities across depths. Although we have yet to document fishing in MCEs in the Philippines, their predominant gear (44% nets, 40% hook and lines; Muallil et al., Reference Muallil, Mamuag, Cababaro, Arceo and Aliño2014) suggest that they can fish beyond 30 m (Dalzell, Reference Dalzell1996). However, fishing at MCEs may carry the risk of fishing gear entanglement or damage, which is unfavourable to fishers as gear loss or repair is costly, reducing their income that is barely enough to sustain their daily needs (Muallil et al., Reference Muallil, Geronimo, Cleland, Cabral, Doctor, Cruz-Trinidad and Aliño2011). The low abundance and biomass of piscivores in the MCEs of LS and Pal, and their low abundances in KIG, probably indicate that these fishes were extracted as piscivores and are usually one of the most vulnerable to fishing mortality (McClanahan & Mangi, Reference McClanahan and Mangi2003). In contrast, relatively less impacted MCE locations in the Philippines such as Apo Reef Natural Park and Abra de Ilog has shown that biomass increases with depth, with predatory fishes from families Lutjanidae, Serranidae, Lethrinidae and Scombridae rather common (Quimpo et al., Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018a, Reference Quimpo, Cabaitan, Olavides, Dumalagan, Munar and Siringan2018b).
In summary, our study shows that 17–25 mean coral genera and 11–17 mean fish species were recorded in the upper MCEs of the eastern SCS, locally known as the WPS, which expanded the information on depth distributions of these known corals and fishes to 40 m. Coral genera and fish species assemblages were more strongly structured by location than by depth, but both variables drive assemblages. Location differences were probably caused by differences in benthic characteristics, fishing efforts, environmental conditions and oceanographic features; while depth differences were probably associated with the similar benthic composition, interconnectivity between SWRs and MCEs, and the fishing efforts between depths. This may suggest that similarities in local-scale factors (e.g. habitat availability, resources, hydrological conditions, fauna motility and fishing efforts) can dampen community structuring between SWRs and MCEs for the three study locations investigated. However, further research is necessary to fully comprehend how much biodiversity the SCS possesses, and whether the weak structuring across depth for coral and fish assemblages can still be observed when a more broad depth spectrum is investigated.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315419000456
Acknowledgements
We are grateful to G. de Guzman, and crew of the research vessel G.T. Velasquez for their invaluable aid during data collection.
Financial support
This study was funded by the Biodiversity Management Bureau, Department of Environment and Natural Resources Philippines through the programme Coastal Assessment for Rehabilitation Enhancement: Capability and Resilience of EcoSystems (CARE-CaDRES), project West Philippine Sea.










