- DMH
dimethylhydrazine
- IBD
inflammatory bowel disease
- IFN
interferon
- iNOS
inducible nitric oxide synthase
- KF
kefir cell-free fraction
- LAB
lactic acid bacteria
- LI
large intenstine
- TNBS
trinitrobenzenesulfonic acid
Yoghurt and colon cancer
Lactic acid bacteria (LAB) are present in many foods such as yoghurt and are frequently used as probiotics to favour some biological functions of the host. Yoghurt has been defined as a coagulated milk product that results from the lactic acid fermentation of milk by Lactobacillus (L.) delbrueckii subsp. bulgaricus and Streptococcus (S.) thermophilus, according to the Codex Alimentarius(1). In addition to the yoghurt starter cultures, the peptides and other substances released during the fermentation process can also be important. The therapeutic effects of yoghurt and LAB against diseases such as cancer, infection and intestinal inflammation have been widely evaluated(Reference Perdigon, Fuller and Raya2, Reference Kato, Fuller and Perdigon3). The immunomodulating and immunostimulating properties of yogurt and fermented milks have also been well documented(Reference de Moreno de Leblanc and Perdigon4, Reference Matar, Valdez and Medina5). The decrease of cancer occurrence by LAB and yoghurt consumption has been described in experimental models. The preventive effect of some probiotics on intestinal carcinogenesis could be associated with changes in the local microbiota since LAB can suppress the bacterial growth that convert procarcinogens into carcinogens, thereby reducing the amount of carcinogens in the intestine(Reference Kato, Yokokura and Mutai6, Reference Hayashi and Ohwaki7).
Long-term yoghurt-fed mice have been shown to maintain β-glucuronidase and nitroreductase activities similar to or lower than the control group mice(Reference de Moreno de LeBlanc and Perdigon8). This outcome found in intestinal enzyme activities was also shown in a colon cancer model when the injection of a carcinogen, such as dimethylhydrazine (DMH), increased the activity of both enzymes, contributing to its mutagenic power. However, DMH injection produced lower enzyme activity levels in cyclically yoghurt-fed mice than in the tumour control group(Reference de Moreno de LeBlanc and Perdigon8) (Table 1). Nevertheless, this effect was not observed when the mice received the non-bacterial fraction of yoghurt (supernatant). These results show that yoghurt bacteria may be involved in the decrease of procarcinogenic enzyme activities and in the intestinal microbial changes(Reference de Moreno de LeBlanc and Perdigon8).
Table 1. Effect of yoghurt feeding and dimethylhydrazine (DMH) carcinogen on β-glucuronidase and nitroreductase activity in the large intestine content of miceFootnote *
(Mean values and standard deviations for five animals)

Y, yoghurt.
a,b,cMeans for each value without a common letter differ significantly (P<0·05).
* Specific enzyme activity is expressed for β-glucuronidase as the μg of phenolphthalein released per minute per mg protein. For nitroreductase, specific activity is expressed as mg of m-aminobenzoic acid formed per h per mg protein. Significant differences were calculated for each period of treatment by comparing the four experimental groups. Y–DMH–Y is the group of mice that received yoghurt continuously for 10 d before DMH injection and they were fed again with yoghurt in a cyclic basis (10 d of yoghurt with 1 week break) after tumour promotion (2 months) and until the end of the experiment (6 months). The Control group did not receive special feeding or treatment. The yoghurt control group received yoghurt cyclically, as well as Y-DMH-Y. The DMH group is the control of the tumour, injected with DMH weekly during 10 weeks but without special feeding.
Another mechanism involved in this anti-tumour effect of yoghurt is the modulation of intestinal immune response. Thus, yoghurt could exert an anti-inflammatory effect different from that induced by an anti-inflammatory drug administration, such as indomethacin. Histological samples have shown that while indomethacin treatment can diminish the large intestine (LI)-infiltrating immune cells, yoghurt feeding induces increased lamina propria-infiltrating immune cells in mice, both in the presence or absence of the carcinogen(Reference de Moreno de LeBlanc, Valdez and Perdigón9). These results led us to study the role of these immune cells in the LI. The analysis of the cytokines produced by these cells showed that the secretion of a pro-inflammatory cytokine such as interferon (IFN)-γ increased in the LI cells from the tumour control mice, yoghurt-fed mice with carcinogen injection (yoghurt–DMH–yoghurt group) and also yoghurt-fed mice without carcinogen injection (Table 2). This outcome was different from that shown with the indomethacin treatment where LI-infiltrating cells and IFN-γ-secreting cells significantly diminished(Reference de Moreno de LeBlanc, Valdez and Perdigón9).
Table 2. Study of pro-inflammatory and regulatory cytokines in the colon cancer model and the inflammatory bowel disease (IBD) modelFootnote *
(Mean values and standard deviations)

DMH, dimethylhydrazine; IFN, interferon; ND, not determined; TNBS, trinitrobenzenesulfonic acid; Y, yoghurt.
abcValues for each cytokine-positive cells without a common letter differ significantly (P<0·05).
* The producer cells for IFN-γ and IL-10 were studied in the large intestine tissues by indirect immunofluorescence technique. The results are expressed as the number of positive cells for the correspondent cytokine in 10 fields of vision as seen at 1000× magnification using a fluorescence light microscope. Y–DMH–Y is the group of mice that received yoghurt continuously for 10 d before DMH injection and they were fed again with yoghurt in a cyclic basis (10 d of yoghurt with 1 week break) after tumour promotion (2 months) and until the end of the experiment (6 months). The Control group did not receive special feeding or treatment. The yoghurt control group received yoghurt cyclically, as well as Y–DMH–Y. The DMH group is the control of the tumour, injected with DMH weekly during 10 weeks but without special feeding.
According to a histological study, and in order to demonstrate that an increase of this pro-inflammatory cytokine in mice fed with yoghurt was not related to an inflammatory response, the number of inducible nitric oxide synthase (iNOS) enzyme producing cells was studied. iNOS, which produces nitric oxide from L-arginin, is induced during the immune response by microbial products and/or cytokines and is also involved in the anti-microbial effector mechanism of macrophages(Reference Bogdan, Rollinghoff and Diefenbach10). One of the T-helper 1 effector mechanisms mediated by IFN-γ is iNOS induction, and IL-4 plays an important role as its regulator(Reference Patton, La Flamme and Pedras-Vasoncelos11). High numbers of iNOS (+) cells have been shown in tumour-bearing mice (DMH group), suggesting an increase in nitric oxide production by these cells. This outcome agrees with the IFN-γ increase observed in mice from this experimental group where this cytokine could be involved in the iNOS enzyme induction. In the yoghurt-fed mice with DMH injection, the number of iNOS (+) cells decreased. The same effect was observed in the long-term yoghurt-fed mice without DMH injection. The lack of iNOS enzyme induction in the yoghurt-fed mice (yoghurt–DMH–yoghurt and yoghurt control groups) showed that yoghurt would regulate the immune system by modulating the inflammatory response. In spite of the increased number of IFN-γ (+) cells, neither nitric oxide production was increased nor was tumour present. In view of these results, we may suggest that the large number of IFN-γ (+) cells in yoghurt-fed mice could be related to the increased number of intestine immune cells and could be regulated by other cytokines such as IL-10.
Moreover, IL-10-secreting cells were analysed in the colon cancer model in order to find out more deeply about the possible regulatory mechanisms induced by yoghurt(Reference Perdigon, de Moreno de LeBlanc and Valdez12). The number of IL-10 (+) cells increased significantly in all samples from the experimental groups: tumour control, mice injected with DMH and fed with yoghurt and yoghurt control group(Reference Perdigon, de Moreno de LeBlanc and Valdez12). It is important to highlight that yoghurt feeding produced by itself the highest number of IL-10 (+) cells, which shows that the administration of this fermented milk contributed to maintain a regulated immune response in the intestine of yoghurt-fed mice (Table 2).
These results suggest that yoghurt feeding modulates the immune response as follows: (1) by stimulating cytokine production when required (as was observed in the tumour model where yoghurt feeding increased the number of positive cells for different cytokines) and (2) by regulating this production in order to prevent an exacerbation of the inflammatory immune response. This effect occurs mainly through IL-10, which increased in the LI tissue during all the periods assayed (2, 4 and 6 months, and 10 d).
Yoghurt and inflammatory bowel diseases
Inflammatory bowel disease (IBD), a chronic immune-mediated disease, which includes Crohn's disease and ulcerative colitis, has been associated with increased colon cancer risk(Reference Langholz, Munkholm and Davidsen13, Reference Gillen, Walmsley and Prior14). The complete aetiology of both diseases is still unknown but genetic, environmental and immunological factors might be involved. However, there is a growing body of evidence that supports the role of intestinal microbiota in IBD initiation and progression(Reference Linskens, Huijsdens and Savelkoul15).
The regulation of the inflammatory immune response is one of the mechanisms by which yoghurt could prevent the risk of colon cancer in experimental models, as described above. That result led us to analyse the effect of this fermented milk in an intestinal inflammation model induced with trinitrobenzenesulfonic acid (TNBS).
An important reduction of the colonic damage caused by TNBS was observed in mice fed yoghurt during 10 d before TNBS and continued yoghurt administration after TNBS inoculation (before and after inflammation)(Reference de Moreno de LeBlanc, Chaves and Perdigón16). Although some mucosa and submucosa infiltrates were present, most of the tissues of the LI were similar to those observed in the control animals that were inoculated with alcohol 50% (TNBS vehicle).
Enzyme activities in the LI of yoghurt-fed mice injected with DMH were reduced. Therefore, we hypothesised that intestinal bacteria composition could have been modified by yoghurt consumption, and we analysed the gut microbiota in the TNBS-induced intestinal inflammation model.
Most of the models in which animals develop colitis are influenced by the microbiota present in the intestinal lumen. This fact is supported by the reduction or absence of intestinal inflammation in TNBS or dextran sulfate sodium models of colitis using antibiotic-treated and germ-free animals(Reference Rath, Wilson and Sartor17–Reference Chandran, Satthaporn and Robins19).
In the yoghurt-fed mice group, the basal sample (after 10 d), on the same day of TNBS inoculation, was compared with the sample obtained from a control group. Changes in the intestinal microbiota were observed; bifidobacteria and total lactobacilli counts were higher in yoghurt-fed mice than in the control group (Table 3). These increases were also maintained in yoghurt-fed mice inoculated with TNBS; however, this result was not found in those mice in which yoghurt administration was stopped after the inflammatory drug inoculation. Thus, there might be a preventive role of yoghurt against this type of inflammation(Reference de Moreno de LeBlanc, Chaves and Perdigón16). Indeed, bifidobacteria and lactobacilli are known to be desirable inhabitants of the intestine and are related to beneficial effects in the gastrointestinal tract, such as the prevention of inflammation. Those changes observed in the intestinal microbiota could explain some of the mechanisms by which yoghurt feeding exerts its anti-inflammatory effect in our IBD model. The TNBS model is associated with the absence of lactobacilli together with an increase in other aerobic isolates such as Escherichia coli and Staphylococcus spp(Reference Videla, Vilaseca and Guarner20). Similarly, decreased levels of faecal lactobacilli and bifidobacteria have also been reported in Crohn's disease in human subjects(Reference Favier, Neut and Mizon21).
Table 3. Effect of yoghurt feeding on the microbiota of the large intestine (LI)Footnote *
(Mean values and standard deviations)
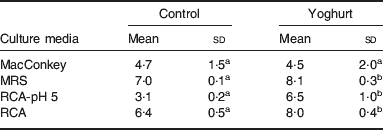
abMean values for each medium without a common letter differ significantly (P<0·05).
* Results are expressed as means with their standard deviation of the log10 CFU/g LI for control mice and mice fed for 10 d with yoghurt (yoghurt group). The content of the LI from each mouse was placed in different agarized media: reinforced clostridial agar, for total anaerobic bacteria; RCA containing 0·2% LiCl, colistin 4 mg/l, 1% aniline blue and after sterilization adjusted to pH 5 with acetic acid (RCA-pH 5) for isolation of bifidobacteria; Mann–Rogosa–Sharp Agar for total lactobacilli and MacConkey for Enterobacteriaceae.
Another characteristic of intestinal inflammation is an increased permeability of the colonic mucosa barrier. All these features contribute to bacterial translocation towards distant organs such as liver and spleen. The bacterial translocation towards the liver has been shown to be lower in mice that received yoghurt and were inoculated with TNBS than in those mice that received only TNBS inoculation(Reference de Moreno de LeBlanc, Chaves and Perdigón16).
This outcome may confirm that yoghurt feeding is capable of diminishing the severity of inflammation in mice, and changes in the intestinal microbiota could be a contributing factor to this beneficial effect.
Considering, on the other hand, that decreased IgA levels could be related to intestinal inflammation and that IgA enhancement has been reported as one of the beneficial effects associated with probiotic consumption, we analysed IgA-secreting cells in the LI of those yoghurt-fed mice with TNBS inoculation. Yoghurt feeding increased the number of IgA (+) cells in those mice inoculated with TNBS in comparison with the TNBS control group(Reference de Moreno de LeBlanc, Chaves and Perdigón16).
These results are in agreement with the IgA increase reported after Lactobacillus rhamnosus GG administration in Crohn's disease(Reference Malin, Suomalainen and Saxelin22) and with previous results where yoghurt administration increased the number of IgA (+) cells in a colon cancer model in mice(Reference Perdigon, de Moreno de LeBlanc and Valdez12). This result could limit the inflammatory response, since IgA is considered an important barrier in colonic neoplasia(Reference de Moreno de LeBlanc, Matar and Perdigon23).
According to previous results where yoghurt feeding showed anti-inflammatory properties by modulating the immune response, the study of cytokine-secreting cells was the logical next step. Cytokines are known to be capable of regulating T-cell proliferation and differentiation and determining the course of an inflammatory process by releasing pro- and anti-inflammatory cytokines. TNBS is an animal model for Crohn's disease with an increased T-helper1:T-helper2 ratio. The suppression or regulation of an exacerbated T-helper1 response could be an alternative for IBD therapy both in animal models and human subjects. Pro-inflammatory cytokines such as IL-12 and IFN-γ are thought to promote the development of these diseases(Reference Fuss, Neurath and Boirivant24).
Yoghurt feeding maintained a high number of IFN-γ (+) cells (Table 2); however, compared to the inflammation control, the fermented milk administration decreased the number of IFN-γ (+) and IL-12 (+) cells, leading to a regulated inflammatory response, which was related to a positive effect of yoghurt against colitis(Reference de Moreno de LeBlanc, Chaves and Perdigón16).
In addition, the recently described IL-17-expressing T-helper cells may play a central role in T-cell-mediated diseases including IBD(Reference Dong25, Reference Zhang, Zheng and Bindas26). It is now a generally accepted concept that IL-17 stimulates the production of inflammatory mediators and it is a key element in the inflammatory cascade in a variety of pathological conditions.
The lower levels of IL-17-secreting cells in the LI of yoghurt-fed mice confirmed the anti-inflammatory effect of yoghurt in the TNBS-induced inflammation model(Reference de Moreno de LeBlanc, Chaves and Perdigón16). However, it is also important to highlight that contrary to TNF-α and IFN-γ increases found after yoghurt feeding, none altered IL-17 production triggered by yoghurt administration.
In addition, IL-10 was studied in the intestinal inflammation model as a regulatory cytokine, considering the critical role of IL-10 as an anti-inflammatory cytokine at the intestinal level(Reference Berg, Davidson and Kuhn27) and the previous results obtained with the pro-inflammatory cytokines where yoghurt administration induced a down-regulated inflammatory response in the colon cancer model.
An increased IL-10-secreting cell number was found in yoghurt-fed mice in comparison with the inflammation control group. This increase was more evident in those mice that received continuous yoghurt administration, even after TNBS inoculation (Table 2). IL-10 secretion could be one of the mechanisms by which yoghurt can exert its anti-inflammatory effect. It has been interestingly reported that the induction of colitis is prevented by co-transfer of syngenic CD4+CD45RBlow T-cells that exert their anti-inflammatory effect via production of IL-10 and transforming growth factor-β(Reference Singh, Read and Asseman28).
The results obtained suggest that yoghurt can modulate the immune response inducing down-regulation of the immune cells involved in the inflammatory process. This effect would occur mainly through IL-10 and by declining IL-17 and IL-12 secretion.
In addition, another mechanism by which yoghurt could exert its anti-inflammatory activity would be through beneficial changes in the intestinal microbiota (increases in bifidobacteria and lactobacilli populations). These changes in the intestinal microbiota could also be considered as one of the possible causes to reduce intestinal inflammation.
Fermented milks and breast cancer
The intestine is the first site to assay different properties of probiotics that enter orally into the host. However, it is important to consider that the host contains the ‘common’ mucosal immune system, which ensures that all mucous membranes are furnished with a wide spectrum of secretory antibodies(Reference Brandtzaeg, Farstad and Haraldsen29). Both B-cells and T-cells can migrate from Peyer's patches, found in the small intestine, to mucosal membranes of the respiratory, gastrointestinal and genitourinary tracts, as well as to the exocrine lacrimal, salivary and prostatic glands(Reference Brandtzaeg and Pabst30). Thus, Lactobacillus casei CRL 431 orally administered has been able to stimulate the IgA cycle, increasing IgA (+) cells, not only in the intestine but also in the bronchus and mammary gland tissues(Reference de Moreno de LeBlanc, Maldonado Galdeano and Chaves31).
The oral administration of milk fermented with L. helveticus R389 (bacteria with high protease and peptidase activity) also increased the number of IgA-secreting cells in the small intestine as well as in the bronchus of mice(Reference LeBlanc, Matar and Valdez32).
Peptide fractions released during milk fermentation with L. helveticus R389 have been reported to stimulate the immune system and inhibit the growth of an immuno-dependent fibrosarcoma in a mouse model, suggesting the importance of the bacteria as well as the products released during milk fermentation(Reference LeBlanc, Matar and Valdez32).
These previous works led us to study the effect of milk fermented with L. helveticus R389 on the growth of breast cancer in the animal model. The effect of kefir and its cell-free fraction (kefir cell-free fraction (KF)) administrations were also determined in this type of cancer model since both kefir and its products possess several substances that can exert beneficial effects on the immune system and prevent certain types of cancer(Reference Vinderola, Duarte and Thangavel33–Reference Murofushi, Mizuguchi and Aibara35).
The independent administration of these three products (L. helveticus R389, kefir and KF) has been shown to exert beneficial effects against breast cancer. However, the administration period that required to have the desirable effect was different for each of them, probably due to the complexity and microbial charge of kefir compared with the fermented milk used in this study. Seven days of cyclical administration of milk fermented with L. helveticus R389 delayed or stopped tumour development. Tumour growth also diminished in mice after receiving 2 d of cyclical feeding with whole kefir, and the same cyclical feeding with KF showed the most significant delay of the tumour growth(Reference de Moreno de LeBlanc, Matar and Farnworth36).
The influence of the immune cells on breast cancer has been reported by using different models(Reference Reome, Hylind and Dutton37, Reference Zhang, Li and Holle38). A substantial proportion (up to 50%) of breast tumours is made up of tumour-infiltrating immune cells(Reference Reed and Purohit39), which produce different biological messengers, such as cytokines, involved in an anti-tumour response. Therefore, different cytokines were analysed in several samples to have a spectrum at a systemic level, and to measure the local response in mammary glands or tumours in order to study the effect of fermented milks on the immune response.
The pattern of cytokines was similar for the three products in relation to the delay of the tumour development.
Serum TNF-α levels showed a time-dependent increase, as did the tumour volume in the control group(Reference de Moreno de LeBlanc, Matar and Farnworth36, Reference de Moreno de LeBlanc, Matar and LeBlanc40). A significant TNF-α increase was shown in those mice cyclically fed with milk fermented with L. helveticus R389, kefir or its KF, compared to the tumour control group. TNF-α-secreting cells in mammary glands showed similar patterns to those levels obtained for this cytokine in serum (Table 4). This increase prior to tumour induction could be related to the decrease in tumour growth; however, it is important to note that TNF-α concentration was maintained or decreased after tumour injection in those mice that received the fermented products. Indeed, this cytokine can be related to oestrogen synthesis in the mammary gland, and thus to the tumour growth(Reference Reed and Purohit39).
Table 4. Study of cytokine-secreting cells in mammary glands and the cytokine concentrations in blood serumFootnote *

abcdefghMean values for each cytokine in tissue or serum without a common letter differ significantly (P<0·05).
* The basal samples were taken on the day of the tumour injection (after 7 d of L. helveticus R389 fermented milk administration or 2 d after kefir or kefir cell-free fraction (KF) administration) and the other samples 12, 18, 22 or 27 d post tumour cell injection. The tumour controls are mice injected with the breast tumour cells (4T1 cell line) without special feeding. Means for each cytokine in tissue or serum without a common letter differ significantly (P<0·05).
† For mammary gland tissues, cytokine-positive cells were analysed using indirect immunofluorescence. The results are expressed as means with their standard deviations of cytokine-positive cells counted in 10 fields of vision at 1000× of magnification.
‡ For blood serum, cytokine concentrations were measured by ELISA. The results are expressed as mean concentration of each cytokine (pg/ml) with their standard deviations.
IL-6 was also determined since it is one of the most important cytokines involved in oestrogen synthesis, a hormone that is essential for tumour growth. The tumour grew at a faster rate in the control group, which showed elevated serum IL-6 levels. However, a decrease of IL-6 was shown in those groups of mice fed with the fermented milks or KF, suggesting an involvement of IL-6 in the tumour growth delay(Reference de Moreno de LeBlanc, Matar and Farnworth36, Reference de Moreno de LeBlanc, Matar and LeBlanc40) (Table 4). Although the knowledge of the systemic immune responses is important, the study of local cytokine responses could be more useful since there is a local oestrogen synthesis in mammary glands, which could be involved in tumour growth. Thus, IL-6 (+) cells significantly increased in mammary glands in the tumour control group throughout the experiment (Table 4). The mice fed with the fermented milk, kefir or its KF maintained low numbers of IL-6 (+) cells over the course of this experiment, which were lower than in the tumour control group(Reference de Moreno de LeBlanc, Matar and Farnworth36, Reference de Moreno de LeBlanc, Matar and LeBlanc40).
IL-10 was also analysed. Both IL-10 serum levels and IL-10-secreting cells in mammary glands were significantly increased in the group of mice where the tumour growth was delayed, which could explain the regulation of the immune response (TNF-α and IL-6). However, this regulatory response was not observed in the tumour control group (Table 4).
The role of tumour-infiltrating immune cells in this anti-tumour response has also been reported in the same mice model(Reference de Moreno de LeBlanc, Matar and Farnworth36, Reference de Moreno de LeBlanc, Matar and LeBlanc40). TNF-α (+) cell numbers showed differences in the isolated cells compared with the other samples (serum and mammary glands). These cells increased in the groups fed with fermented products and KF where the tumour growth was delayed, leading to an induction of TNF-α production by fermented milk, which may play a biological role in the induction of cellular apoptosis, also increased in these animals(Reference de Moreno de LeBlanc, Matar and LeBlanc40). IL-10 is a regulatory cytokine that can be released by tumour-infiltrating immune cells such as macrophages and lymphocytes. IL-10 (+) cell numbers decreased in the tumour control group throughout the time of the study. These cells increased significantly in those mice fed with fermented products or KF. This outcome could occur in order to regulate the pro-inflammatory cytokine (TNF-α and IL-6) productions. Decreased number of IL-6 (+) cells was shown in those mice fed with fermented milks compared to the tumour control group. This outcome shows a protective effect of LAB or other products released during the milk fermentation in this oestrogen-dependent tumour, mainly due to the decrease in IL-6 (Table 5).
Table 5. Production of cytokines by the tumour-infiltrating immune cellsFootnote *
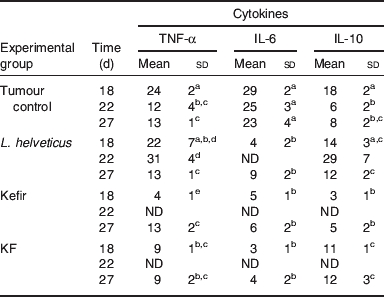
ND, not determined
abcdMeans for each cytokine without a common letter differ significantly (P<0·05).
* Cytokine-positive cells were analysed by using the immunoperoxidase technique in the immune cells isolated from the breast tumour. The results are expressed as means with their standard deviation of cytokine-positive cells per 100 counted cells (cells/100). The samples were taken 18, 22 and 27 d after tumour cell injection. The tumour controls are mice injected with the breast tumour cells (4T1 cell line) without special feeding. L. helveticus, kefir and kefir cell-free fraction (KF) groups are mice that received 7 d of L. helveticus R389 fermented milk administration or 2 d of kefir or KF administration, respectively; after these periods they were injected with the tumour cells and continued to receive the administration of the correspondent product for each group in a cyclical basis with 1 week of break.
In view of these results, we can conclude that in the breast cancer model assayed, mucosal sites, even those distant to the intestine, can be stimulated by administrating fermented products. Both probiotic strains and whole fermented products could play an important role in the mucosal activation. A short period of cyclical administration of kefir and KF was sufficient to have the same protective effect as the one observed by using a longer period of cyclical feeding with the milk fermented with L. helveticus R389. This effect seemed to be mainly related to IL-6 decreases. KF and the milk fermented with L. helveticus R 389 induced not only IL-6 decrease but also exerted a regulatory response by increasing IL-10 levels.
The results obtained have shown that the effect in this tumour model could be not only due to micro-organisms but also due to substances released during milk fermentation. This outcome was observed when kefir was compared to KF(Reference de Moreno de LeBlanc, Matar and Farnworth36) and when the milk fermented with L. helveticus R389 was compared to a milk fermented with a non-proteolytic mutant strain of L. helveticus (Reference de Moreno de LeBlanc, Matar and LeBlanc40).
The adequate balance between systemic and local immune responses to delay the tumour growth in mammary glands was also shown.
We have demonstrated that the consumption of fermented milks can modulate the mucosal immune system in murine models. The administration of fermented products may exert an influence on the intestinal microbiota, stimulate the intestine-associated immune cells and maintain a regulated immune response that is useful against the intestinal inflammation and also against colon cancer. The oral administration of fermented products can also be beneficial to combat other pathologies in tissues distant from the intestine, as it was already observed for the breast cancer model. Unlike the intestinal immune stimulation, the effect in the mammary glands was observed only when a local stimulus, such as tumour cells, was present.
It is important to remark that fermented milk consumption can stimulate the immune system and can maintain it in a state of surveillance, which could be useful to affront different pathologies such as cancer and intestinal inflammation.
Acknowledgements
This study was supported financially by Agencia Nacional de Promoción Científica y Tecnológica (ANPyCT-FONCYT, PICT 05/33213), Consejo de Investigación de la Universidad Nacional de Tucumán (CIUNT 26/D 442) and Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET, PIP 0652), Argentina. The authors declare no conflict of interest. Both authors conceived, designed and carried out animal studies, performed the statistical analyses, prepared the tables and wrote the manuscript.







