Breast cancer remains a significant health burden in both developed and developing countries, with over 1·68 million cases diagnosed globally in 2012(Reference Ferlay, Soerjomataram and Ervik1). Although, in many countries, the number of breast cancer survivors is growing due to earlier diagnosis and advances in treatment, this diagnosis is still the most common cause of cancer death worldwide for females, accounting for 522 000 deaths in 2012(Reference Ferlay, Soerjomataram and Ervik1). In the UK, there are over 55 000 new breast cancer diagnoses and over 12 000 deaths due to this disease per year(2). A number of associations with the development of breast cancer have been identified such as age, early menarche, late menopause, family history and hereditary breast cancer susceptibility syndromes(Reference Rizzolo, Silvestri and Falchetti3). More recently, attention has focused on modifiable risk factors, which describe a range of behavioural and lifestyle factors. The most significant modifiable risk factor for developing breast cancer is obesity, but only convincingly for post-menopausal women(4, 5).
There is now evidence to suggest that obesity at diagnosis of early (curable) breast cancer is associated with reduced breast cancer survival in both pre- and post-menopausal women(Reference Chan, Vieira and Aune6, 7) (Table 1). Here we review the evidence that body mass has implications both for the intrinsic pathology of breast cancer and the effectiveness of treatments, as well as the limitations of the current evidence base. All data presented in this review refer to patients with early breast cancer and not to patients with metastatic disease.
Table 1. Key findings of published meta-analyses and large studies (n > 1000) reporting the effect of obesity at diagnosis on the outcome of early breast cancer
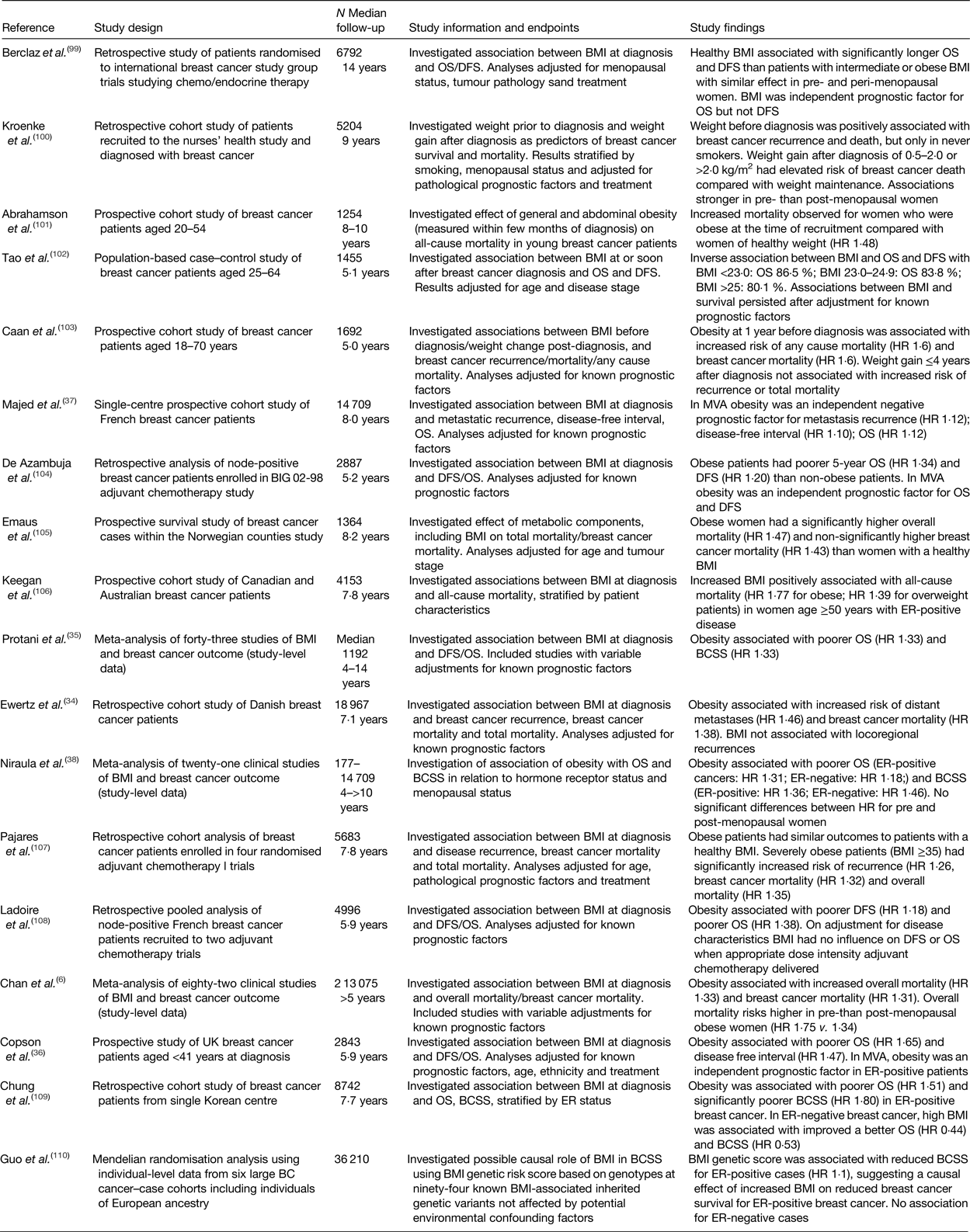
OS, overall survival; DFS, disease-free survival; HR, hazard ratio; MVA, multi-variable analyses; BCSS, breast cancer-specific death; ER, oestrogen receptor.
Obesity: the scale of the problem
Obesity is defined as an excess accumulation of adipose tissue and occurs when energetic intake exceeds energy expenditure. The WHO specifically defines obesity as BMI >30 kg/m2, with BMI 25≤BMI<30 kg/m2, categorised as overweight(8). Storing excess energy served a valuable evolutionary purpose to allow our ancestors to store energy in times of nutritional deprivation. However, obesity is now a pandemic health concern in both developed and developing countries, with over 600 million adults worldwide estimated to be obese(9). In the UK, 27 % of British women are obese and this figure is predicted to increase to 43 % by 2030(10, Reference Wang, McPherson and Marsh11). The links with type 2 diabetes and CVD have long been established, but it has also become increasingly clear that there is a substantial link between obesity and an increased frequency of a number of cancers(Reference Renehan, Tyson and Egger12). The International Agency for Research on Cancer has identified thirteen obesity-related cancers, which include oesophageal adenocarcinoma, stomach, colorectal, liver, gallbladder, pancreas, post-menopausal breast, endometrium, ovary, renal cell, thyroid, meningioma and multiple myeloma(Reference Lauby-Secretan, Scoccianti and Loomis13). Therefore, for these types of cancer, the proportion of patients with obesity is likely to be significant. These associations are further worrying given the increasing prevalence of childhood obesity, potentially increasing the incidence of cancer as these obese children reach adulthood(Reference Jeffreys, Smith and Martin14).
Obesity and risk of breast cancer
The World Cancer Research Fund (WCRF) review has classified the evidence that body fatness increases the relative risk of post-menopausal breast cancer as convincing(5). This risk is of the order of 12 % higher for women who are overweight (BMI 25–29·9 kg/m2) and 16 % higher who are obese (BMI ≥30 kg/m2), compared with women of healthy body mass (BMI 20–24·9 kg/m2)(Reference Cheraghi, Poorolajal and Hashem15). For every 5 kg/m2 increase in BMI, there is a 12 % increased relative risk of post-menopausal breast cancer(Reference Renehan, Tyson and Egger12). Obese post-menopausal women have an increased risk of hormone receptor-positive breast cancer compared with women of healthy BMI; however, obesity is not associated with an increased risk of hormone receptor-negative breast cancer in post-menopausal women(Reference Munsell, Sprague and Berry16).
Post-menopausal hormone replacement therapy may modify the relationship between obesity and post-menopausal breast cancer. The Nurses’ Health Study found that BMI and adult weight gain were associated with 60 % to 2-fold increased relative risk, respectively, of post-menopausal breast cancer among women who had never used hormone replacement therapy, whereas there was an attenuation of this relationship among women who used hormones(Reference Huang, Hankinson and Colditz17). In terms of women who used hormone therapy, results from the Women's Health Initiative showed an increased risk of post-menopausal breast cancer for oestrogen–progestogen preparations compared with oestrogen alone(Reference Morimoto, White and Chen18). The attenuation of the risk seen in women who use hormone replacement therapy may be due to the fact that circulating oestrogen levels are elevated among women using hormone replacement therapy, minimising the impact of the adipose tissue oestrogen production, which is the primary source of oestrogen production via the aromatase enzyme in post-menopausal women.
In contrast, studies indicate that obesity does not increase the risk of developing premenopausal breast cancer with some meta-analyses even suggesting that the risk is slightly lower in pre-menopausal women who are overweight and obese compared with healthy weight individuals(5, Reference Renehan, Tyson and Egger12, Reference Cheraghi, Poorolajal and Hashem15, Reference Chen, Liu and Zhou19). However, weight gain during adulthood is associated with a significant increased risk of post-menopausal breast cancer(Reference Keum, Greenwood and Lee20).
Mechanisms of cancer development in obesity
The molecular mechanisms promoting cancer development in obesity are not fully understood; however, it is clear that a number of different biological pathways are involved(Reference Khandekar, Cohen and Spiegelman21). In addition to storing excess energy in the form of lipid, adipose tissue has an active role in endocrine signalling to the rest of the body. Increased adipose tissue is associated directly with increased levels of many circulating hormones, including insulin, insulin-like growth factors and sex hormones, creating an environment that encourages carcinogenesis(Reference De Pergola and Silvestris22). Abdominal fatness is also associated with insulin resistance, further increasing the pancreatic production of insulin(Reference Gunter, Hoover and Yu23). In addition, extensive data suggest that adipose tissue secretes specific molecules into the bloodstream, which signal to other organs to coordinate responses to an altered metabolic state(Reference Khandekar, Cohen and Spiegelman21). One example is leptin, an adipocyte-derived hormone that is the central mediator of a feedback loop that regulates appetite and energy homeostasis(Reference Jardé, Perrier and Vasson24). There is an up-regulation of the leptin receptor in breast cancer, and leptin also stimulates the expression and activity of aromatase and the transactivation of the oestrogen receptor (ER) in breast cancer cells, both of which stimulate tumour growth(Reference Jardé, Perrier and Vasson24–Reference Catalano, Mauro and Marsico26). Several studies indicate an anti-tumour effect of adiponectin(Reference Jardé, Perrier and Vasson24). Adiponectin levels are reduced in obesity and inversely associated with breast cancer risk in post-menopausal women(Reference Ruan, Zarnowski and Cushman27).
More recently, obesity has become recognised as a chronic pro-inflammatory state.
Cytokines secreted by adipose tissue can activate macrophages, and in obese individuals, adipose tissue becomes infiltrated with macrophages(Reference Weisberg, McCann and Desai28, Reference Xu, Barnes and Yang29). It has been demonstrated that tumour-associated macrophages have a key role in the tumour micro-environment. Increased macrophage chemoattractant protein 1 in breast tumour extracts is an early predictor of early relapse and metastasis(Reference Qian, Li and Zhang30, Reference Ueno, Toi and Saji31). Proliferating macrophages in breast tumours are also associated with a high tumour grade and poor prognosis(Reference Campbell, Tonlaar and Garwood32). Adipocytes are also able to produce pro-inflammatory factors such as TNF-α, C-reactive protein and IL-6, and higher serum levels of these cytokines are seen in obese than lean individuals suggesting that obesity has a systemic as well as local pro-inflammatory effect(Reference De Pergola and Silvestris22). It has been proposed that carcinogenesis is promoted by the activation of the inflammatory cascade(Reference Calle and Kaaks33) (Fig. 1).
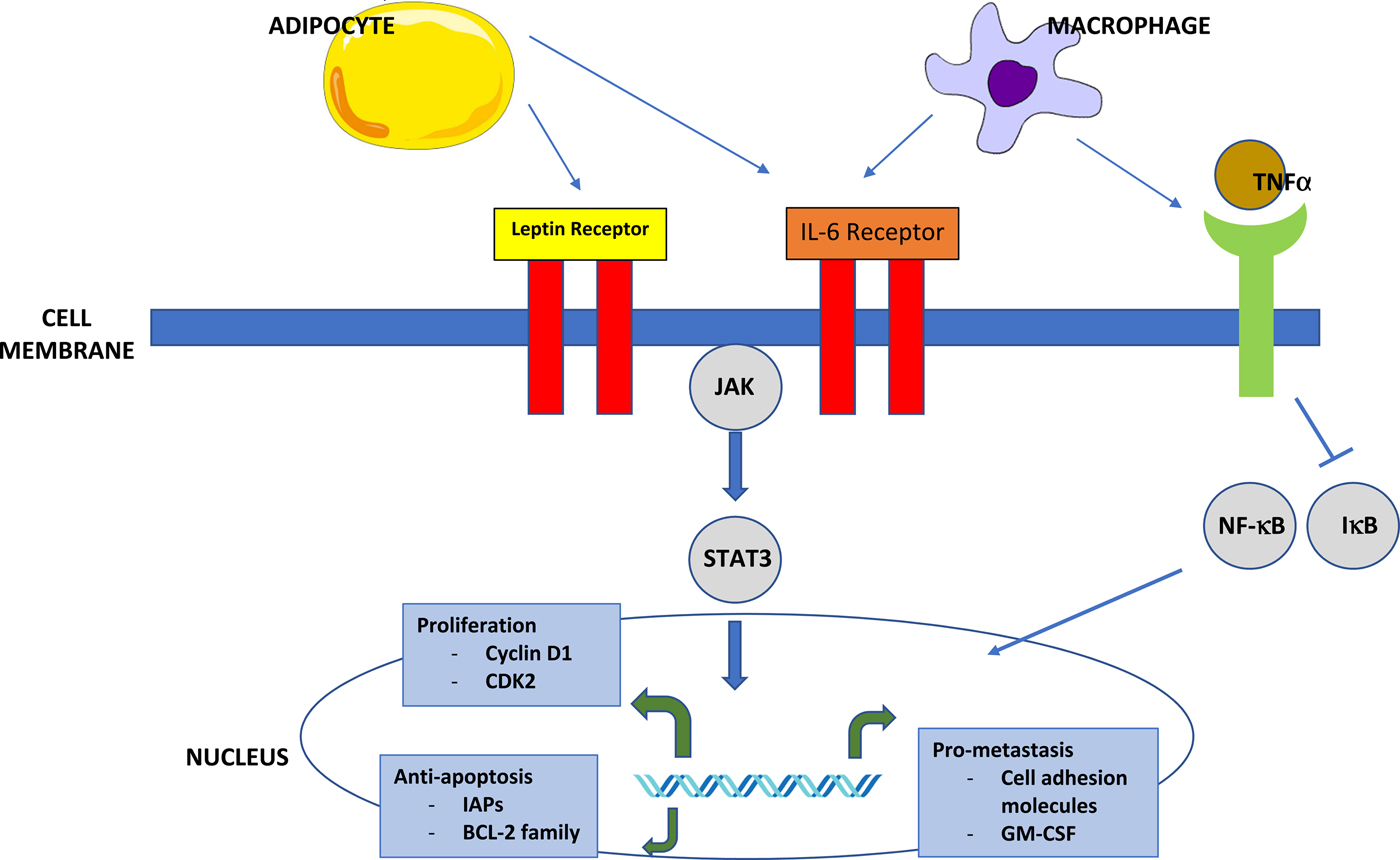
Fig. 1. (Colour online) Inflammatory signalling in obesity. Circulating leptin produced by adipocytes can bind both to the leptin receptor and the IL-6 receptor. This leads to the activation of the JAK-signal transducer and activation of the transcription (STAT) signalling pathway through STAT3. STAT3 functions as an oncogenic transcription factor. Inflammatory cells in the adipose tissue produce IL-6 and TNF-α. IL-6 promotes proliferation and metastasis by activating the JAK–STAT pathway. TNF-α binds to the TNF receptor, activating NF-κB through the degradation of IκB. NF-κB is free to translocate to the nucleus, where it inhibits apoptosis and promotes proliferation and metastasis. Similarly, macrophages are also able to activate the IL-6 and TNF receptors(Reference Khandekar, Cohen and Spiegelman21).
Clearer elucidation of the complex molecular mechanisms that underlie tumour development in obesity is required and may unlock the potential for therapeutic interventions, notably in the modulation of inflammatory pathways as chemoprevention.
Obesity and breast cancer outcomes
Several published meta-analyses indicate that obesity is a prognostic factor for poorer outcomes after a diagnosis of breast cancer(Reference Chan, Vieira and Aune6, Reference Ewertz, Jensen and Gunnarsdottir34, Reference Protani, Coory and Martin35). In the largest of these, Chan et al. reported a meta-analysis of eighty-two clinical studies including 213 075 breast cancer survivors. A pre-diagnosis BMI ≥30 kg/m2 was associated with a total mortality of relative risk 1·41 (95 % CI 1·29, 1·53) compared with a reference group of healthy weight patients (BMI 20–24·9); the relative risk for BMI 25–29·9 was also significantly raised at 1·07 (95 % CI 1·02, 1·12)(Reference Chan, Vieira and Aune6). Each additional 5 kg/m2 BMI before diagnosis was associated with a 17 % increase in total mortality and a 18 % increase in breast cancer mortality (11 % increase in total mortality and 14 % increase in breast cancer mortality at <12 months from diagnosis and 8 and 29 %, respectively, at >12 months from diagnosis)(Reference Chan, Vieira and Aune6). The long-term effects of obesity at diagnosis were highlighted by a study of just under 19 000 Danish women treated for early-stage breast cancer between 1977 and 2006, in which Ewertz et al. found that the risk of distant metastases separated after approximately 3 years, showing a trend of increasing risk with increasing BMI. At 10 years, the incidences were 20·1 % for patients with BMI <25 kg/m2, 22·4 % for patients with BMI 25–29 kg/m2 and 24·3 % for patients with BMI ≥30 kg/m2. At 30 years, the cumulative risks of dying of breast cancer were 46·4 % for patients with BMI <25 kg/m2, 53·4 % for patients with BMI 25–29 kg/m2 and 57·2 % for patients with BMI ≥30 kg/m2(Reference Ewertz, Jensen and Gunnarsdottir34).
Despite the significant quantity of generally consistent data linking obesity at diagnosis of early breast cancer to poorer overall mortality and breast cancer-specific mortality, the 2014 WCRF continuous update project on breast cancer survivors report categorised the level of evidence as limited on the basis it was not clear to what extent individual studies have fully adjusted for potential confounders such as the tumour type, type of treatment, amount of treatment received and the dissemination of the disease(7) (Table 2).
Table 2. World Cancer Research Fund continuous update project on diet, nutrition, physical activity and breast cancer survivors, 2014; summary of panel judgements(7)*
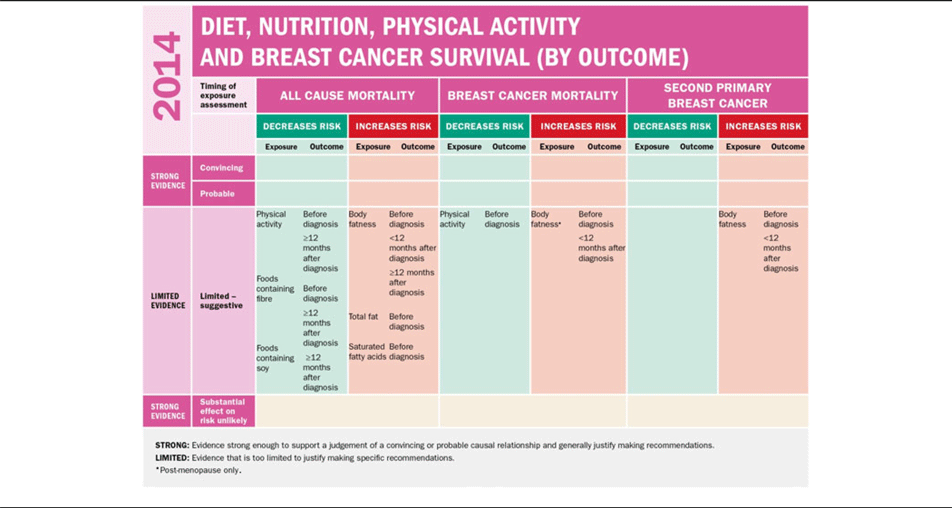
* This material has been reproduced from the World Cancer Research Fund International/Americal Institute for cancer research continuous update project report: diet, nutrition, physical activity and breast cancer surivivors (2014). Available at www.wcrf.org/int/research-we-fund/continous-update-project-reports/breast-cancer-survivors.
Despite the absence of an association between obesity and the risk of developing breast cancer in pre-menopausal women, there is evidence that obesity is significantly associated with poor outcomes in pre-menopausal breast cancer patients. The UK Prospective Study of Sporadic and Hereditary breast cancer in young women, a cohort study of almost 3000 women aged 40 years and under at diagnosis found that obese patients have significantly lower 8-year overall survival than healthy weight patients (58·6 v. 73·3 %, P < 0·001)(Reference Copson, Cutress and Maishman36). Multivariable analyses adjusting for tumour grade, size, nodal and human epidermal growth factor receptor 2 status indicated that obesity was a significant independent predictor of overall survival (hazard ratio 1·65, P < 001) and distant disease-free survival (hazard ratio 1·44). The Chan et al. meta-analysis confirmed that the impact of obesity at diagnosis is greater on pre-menopausal women than post-menopausal women with obesity associated with summary relative risks of 1·75 (95 % CI 1·26, 2·41) for pre-menopausal and 1·34 (95 % CI 1·18, 1·53) for post-menopausal breast cancer(Reference Chan, Vieira and Aune6).
Presentation and pathology
Compared with those with BMI <25 kg/m2, patients with BMI ≥30 kg/m2 tend to have larger tumours and more positive axillary lymph nodes involvement(Reference Ewertz, Jensen and Gunnarsdottir34, Reference Majed, Moreau and Senouci37). These unfavourable pathological features could be explained by a delayed presentation in this group of patients: a larger body habitus may result in breast lumps being less palpable or obvious. More recently, the UK Prospective study of Sporadic and Hereditary breast cancer in young women reported on young breast cancer patients who were below screening age and therefore all presented symptomatically. This study showed that again, in obese and overweight patients, median tumour size was significantly higher and there were more node-positive tumours than in healthy weight patients(Reference Copson, Cutress and Maishman36). In these cohort studies, multivariate analysis demonstrated that obesity retained an independent effect on prognosis on adjustment for disease stage.
Additionally, a number of studies have also reported increased frequency of grade 3 tumours in obese breast cancer patients, compared with those with a healthy weight(Reference Ewertz, Jensen and Gunnarsdottir34, Reference Majed, Moreau and Senouci37), and increased frequency of ER-negative and oestrogen/progesterone/human epidermal growth factor receptor 2 (triple) negative tumours has also been demonstrated(Reference Copson, Cutress and Maishman36). These features are all well-established biomarkers of aggressive behaviour in early breast cancer and these associations suggest that obesity may influence the intrinsic biology of breast tumours, perhaps by affecting the tumour micro-environment. Studies which have adjusted for grade and human epidermal growth factor receptor 2 status suggest that obesity exerts an additional effect beyond these prognostic factors(Reference Ewertz, Jensen and Gunnarsdottir34, Reference Copson, Cutress and Maishman36, Reference Majed, Moreau and Senouci37). Data on the effect of obesity in ER-positive and ER-negative tumours are more conflicting. Although some reports have indicated an equivalent effect of obesity on the outcome of ER-positive and ER-negative tumours(Reference Niraula, Ocana and Ennis38), more recent studies have reported a stronger effect of obesity on the outcome of women with ER-positive breast tumours than ER-negative tumours(Reference Copson, Cutress and Maishman36, Reference Pan and Gray39, Reference Sparano, Wang and Zhao40). Pan et al. demonstrated a breast cancer mortality-adjusted relative risk of 1·34 (95 % CI 1·22, 1·47) associated with obesity in peri/pre-menopausal ER-positive patients but no association between BMI and breast cancer mortality in ER-negative patients(Reference Pan and Gray39, Reference Sparano, Wang and Zhao40). However, it should be noted that many data sets contained relatively small numbers of ER-negative patients and analyses may have been underpowered in this patient group.
Local treatment issues
Surgery
Obese patients with breast cancer have a higher risk of post-surgical complications and this is most clearly seen with post-mastectomy skin flap necrosis where obesity is a recognised risk factor(Reference Robertson, Jeeveratnam and Agrawal41, Reference Robertson, Rusby and Cutress42). Data from the 2007–2012 American College of Surgeons National Surgical Quality Improvement Program® database of 7207 women who had undergone unilateral mastectomy showed a clear increase in both major complications (P = 0·005) and minor complications (P < 0·001) as BMI increased(Reference Garland, Hsu and Clark43). The authors concluded that these findings highlight the need for personalised pre-operative risk assessment and counselling of obese patients(Reference Garland, Hsu and Clark43).
In a series of 718 surgical breast reconstructions including 64 (9 %) obese patients, a number of complications were seen more frequently in obese patients than normal-weight patients including flap complications (39 v. 20 %), overall donor site complications (23 v. 11 %), infection (5 v. 1 %), seroma (9 v. 1 %) and hernia (6 v. 2 %)(Reference Chang, Wang and Robb44). A prospective, multi-centred cohort study of 15 937 patients undergoing breast reconstruction procedures additionally reported that obese patients had greater lengths of hospital stay and longer operating times as well as higher rates of complications (wound, medical, infections, return to theatre, graft loss and major surgical complications). All these factors reached statistical significance(Reference Fischer, Nelson and Kovach45). Furthermore, obesity may negatively influence the decision for immediate breast reconstruction after mastectomy(Reference Fischer, Nelson and Kovach45).
With breast-conserving treatment, there is also increased risk of arm lymphoedema associated with obesity; in a series of 282 patients, higher BMI was related with a greater frequency (36 % of obese patients v. 12 % in the non-obese group) and severity of arm oedema (9 % of obese patients v. 2 % in non-obese patients)(Reference Werner, McCormick and Petrek46). These post-operative complications can all impact on adjuvant treatment, resulting in patients experiencing delays in commencing radiotherapy or chemotherapy.
An interesting observation from the STARSurgUK study, which analysed the surgical outcomes of patients with gastrointestinal cancer, was that there were no effects of increasing BMI on complications after surgery for benign disease, but clear relationships between BMI and post-surgical complications where the indication was malignancy(47). This raises the possibility that the cancer state interacts with obesity to affect the healing process.
Radiotherapy
Use of standard radiotherapy regimens for the breast and chest wall is associated with an increased risk of skin toxicity for the overweight patient(Reference Rodriguez-Gil, Takita and Wright48). Obesity also poses significant challenges in the delivery and accuracy of radiotherapy, notably regarding technical considerations of delivering the optimum radiation dose to the tumour bed. This may require adaptations to equipment, additional time, changes in the posture of the patient and challenges in terms of daily reproducibility(Reference Kubo49–Reference Mohiuddin, Zhang and Tkaczuk51). A particular example is the use of prone breast irradiation for morbidly obese patients. In a study of 110 patients with BMI 34 kg/m2 treated with breast conserving surgery followed by three-dimensional-conformal whole breast irradiation in prone position showed favourable toxicity profiles and good cosmesis(Reference Ergom, Kelly and Morrow52). However, as the standard treatment involves a supine position, there is a clearly a need to prospectively assess tumour dose delivery in obese women(Reference Kirby, Evans and Helyer53).
Systemic treatment issues
Cytotoxic therapy
In adult patients with cancer, chemotherapy dosing has traditionally been based on a patient's estimated body surface area (BSA), calculated from their height and weight(Reference DuBois and DuBois54). Chemotherapy is given either before (neoadjuvant) or after (adjuvant) surgery to selected patients with early breast cancer in order to reduce the risk of future metastatic disease. It is well established that administering adjuvant chemotherapy on time and at the optimal dose is vital to achieve maximal risk reduction with the classic publication by Belladonna indicating that a chemotherapy dose intensity <85 % of the intended dose intensity was equivalent to not giving any chemotherapy at all(Reference Bonadonna, Moliterni and Zambetti55). However, concerns about the potential toxicity of cytotoxic doses associated with high calculated BSA have led to many oncologists routinely capping chemotherapy doses at a maximum BSA of 2·0 or using either ideal body weight or adjusted ideal body weight to calculate drug doses. Reviews of routine practice indicate that up to 40 % of obese patients receive limited doses that are not based on actual body weight and it has been postulated that this use of sub-optimal chemotherapy doses results in a reduced therapeutic effect, which could potentially explain the observed adverse prognosis of obese patients(Reference Lyman, Dale and Crawford56–Reference Chan, Vieira and Aune60).
An expert panel review of the literature found no evidence that short- or long-term toxicity is increased in obese patients receiving full weight-based chemotherapy doses and the resultant American Society of Clinical Oncology clinical practice guideline recommends that full weight-based cytotoxic chemotherapy doses be used to treat obese patients with cancer(Reference Griggs, Mangu and Anderson61) (Table 3). However, it should be noted that the data presented were taken largely from clinical trial patients and it is possible that patients in trials have been selected for a certain level of medical fitness; therefore, these data may not fully represent the real-world situation. In addition, the data collected largely looked only at haematological toxicity criteria. The need for more research in this area was therefore highlighted by American Society of Clinical Oncology(Reference Griggs, Mangu and Anderson61). A subsequent meta-analysis of 9134 chemotherapy patients treated on the basis of actual body weight found similar or lower rates of toxic effects compared with normal-weight patients(Reference Hourdequin, Schpero and McKenna62). However, a more recent study of data from 3023 patients with breast cancer published after this did demonstrate an increase in significant toxicity in obese patients given dose-intense anthracycline and taxane chemotherapy regimens dosed according to actual body weight(Reference Furlanetto, Eiermann and Marme63).
Table 3. Summary of American Society of Clinical Oncologists panel recommendations for treatment of obese patients(Reference Field, Kosmider and Jefford59)
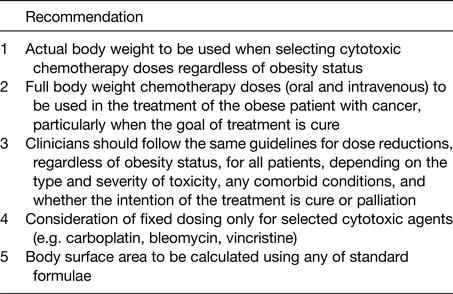
The consequences of a high proportion of obese patients being treated with reduced doses are that survival advantages are lost and outcomes are potentially similar to those of the untreated cohorts. This is seen in trials (of different cancer types including breast) where participants received reduced doses of adjuvant chemotherapy, and where the adjuvant chemotherapy is known to improve survival(Reference Wildiers and Reiser64, Reference Budman, Berry and Cirrincione65). It may also be the case that the observed adverse prognosis of obese patients may be the result of the sub-therapeutic chemotherapy doses and reduced therapeutic effect rather than obesity as a stand-alone factor(Reference Hunter, Navo and Thaker66).
Studies of pathological response rates in the neoadjuvant treatment setting offer to provide a further insight into the effect of obesity on the effectiveness of systemic therapies; however, currently the available data are conflicting. Although a number of studies have reported higher BMI associated with poorer pathological complete response rates and inferior overall survival(Reference Litton, Gonzalez-Angulo and Warneke67–Reference Fontanella, Lederer and Gade69), other studies have not found this association(Reference Farr, Stolz and Baumann70). This may also be explained by variations in chemotherapy prescribing habits as Farr et al.(Reference Farr, Stolz and Baumann70) confirmed that all obese patients analysed had received full uncapped doses of anthracycline–taxane-based chemotherapy whilst prescribing data were not available in all the other publications.
Biological therapies
Unlike traditional cytotoxic drugs, most biological anti-cancer agents (including monoclonal antibodies and small molecule inhibitors) are prescribed by weight or as absolute doses. The most commonly used biological agent in early breast cancer treatment is trastuzumab (Herceptin). This is currently prescribed as either a flat dose (600 mg) for the subcutaneous formulation, or at a maintenance dose (6 mg/kg) for the intravenous preparations; there is no specific guidance for the dosing of obese patients, although in some but not all studies, obesity was found to be a risk factor for trastuzumab-induced cardiac toxicity(71, Reference Jawa, Perez and Garlie72).
Hormonal therapies
The observation that obesity has a greater impact on the prognosis of ER-positive than ER-negative patients(Reference Copson, Cutress and Maishman36, Reference Pan and Gray39, Reference Sparano, Wang and Zhao40) could indicate that obesity influences the effectiveness of adjuvant hormonal therapy effectiveness. Concerns have been reported that aromatase inhibitors (AI; used commonly as adjuvant treatment in post-menopausal breast cancer patients) may be less effective in obese than healthy weight patients as obesity is associated with increased levels of peripheral aromatase activity, which is the therapeutic target for AI. An analysis of the ABCSG-12 trial reported that BMI significantly influenced the efficacy of (the AI) anastrozole plus goserelin in premenopausal patients but did not influence the prognosis of patients treated with tamoxifen plus goserelin(Reference Pfeiler, Konigsberg and Fesl73). However, analyses of interactions between AI and BMI in other breast cancer trials have been inconsistent in terms of long-term outcomes(Reference Goodwin74). Goodwin and co-workers concluded that, overall, the findings did not support the use of BMI as a prediction biomarker of treatment with AI in the adjuvant setting in post-menopausal women with breast cancer(Reference Goodwin74–Reference Weiden, Mackell and McDonnell76). Associations between non-adherence and BMI have been reported for other medications but data on tamoxifen adherence and BMI are lacking(Reference Makari-Judson, Judson and Mertens77).
Weight changes during treatment for breast cancer
Weight gain during chemotherapy is a common feature of treatment with contemporary regimens of 4–6 months of anthracycline and taxane chemotherapy, which are the standard regimens for early breast cancer. European, American and Asian studies report that 30–50 % of women gain more than 5 % of body weight, with a mean weight change of 2–3 kg in the first year after diagnosis(Reference Makari-Judson, Judson and Mertens77–Reference Renehan, Harvie and Cutress88). There is a persistence of weight gain when measured at 3 and 6 years after diagnosis(Reference Lankester, Phillips and Lawton78, Reference Harvie, Campbell and Baildam85). Weight is gained both during and after the chemotherapy period(Reference Freedman, Aziz and Albanes83, Reference Harvie, Campbell and Baildam85). The cause of weight gain due to chemotherapy is usually multifactorial, with use of steroids, change in eating patterns, reduction in physical activity due to fatigue, oedema secondary to taxane chemotherapy and hormonal changes all playing a role. The greatest weight gain is observed in women who are premenopausal, stop smoking after the diagnosis, have a healthy weight at diagnosis or experience a chemotherapy-induced menopause(Reference Makari-Judson, Judson and Mertens77, Reference Freedman, Aziz and Albanes83, Reference Sedjo, Byers and Ganz86, Reference Goodwin, Ennis and Pritchard87). Studies indicate changes in body composition with gain of fat mass and loss of muscle as well as alterations in fluid compartments(Reference Renehan, Harvie and Cutress88).
The consequences of chemotherapy-related weight gain was addressed by Playdon et al. in their meta-analysis of 23 832 cancer cases where they found that, compared with women who maintained their weight, those women with a >10 % weight gain after diagnosis had increased overall mortality (hazard ratio 1·23; 95 % CI 1·09, 1·39, P < 0·001); breast cancer-specific mortality was increased but not to a statistically significant level (hazard ratio 1·17; 95 % CI 1·00, 1·38, P = 0·05)(Reference Playdon, Bracken and Sanft89). No association between weight gain and recurrence was identified. In contrast, in a pooled analysis of 6596 women with ER-positive tumours, more than 10 % weight gain was associated with an increased risk of late recurrence, defined as more than 5 years after diagnosis(Reference Nechuta, Chen and Cai90).
Limitations of using BMI
BMI is a simple surrogate marker of obesity, but it is not a reliable measure of body fat for individuals as it cannot distinguish lean mass from fat mass, or characterise body fat distribution. Body fat is generally distributed viscerally, subcutaneously and internally (most in the liver), and the body fat distribution pattern can differ significantly between individuals with the same BMI. Similarly, patients with the same BSA may have wide variations in the distribution of adipose tissue and skeletal muscle; thus, BSA fails to accurately reflect drug pharmacodynamics and pharmacokinetic variability in obese patients. In a large analysis of 1206 cancer patients, Sparreboom et al. showed drug-specific interactions between BMI and the pharmacokinetic clearance of cytotoxic drugs including doxorubicin, which is commonly used in the treatment of early breast cancer(Reference Sparreboom, Wolff and Mathijssen91).
Very few studies have looked at the effect of body composition patterns on chemotherapy tolerance because the gold standard ‘four-compartment model’ measurement of body composition requires complex procedures not routinely available in the clinical environment. Two small series using limited body composition data derived from comprised tomography images indicate that sarcopaenia (low muscle mass) is associated with increased chemotherapy toxicity, regardless of overall BMI(Reference Prado, Baracos and McCargar92, Reference Prado, Antoun and Sawyer93). Sarcopaenia may result in a relatively higher chemotherapy dose as lean body mass is a predictor of volume of distribution for some drugs(Reference Morgan and Bray94). Del Fabbro et al. reported a higher pathological complete response rate to neoadjuvant chemotherapy for breast cancer in healthy BMI patients with sarcopaenia(Reference Del Fabbro, Parsons and Warneke95).
In a larger study, Iwase et al. retrospectively analysed 172 advanced breast cancer patients who underwent surgery after neoadjvuant chemotherapy(Reference Iwase, Sangai and Nagashima96). Body composition parameters including abdominal circumference, subcutaneous fat area, visceral fat area and skeletal muscle area were calculated using comprised tomography volume-analysing software. Distant disease-free survival was significantly worse in the high visceral fat area group than in the lower visceral fat area group. Furthermore, in the high visceral fat area group, post-menopausal patients had significantly shorter distant disease-free survival than premenopausal patients(Reference Del Fabbro, Parsons and Warneke95). The importance of visceral fat may be explained by the fact that it appears to be more hormonally active than other types of body fat; this appears to promote inflammation, insulin resistance and increases in leptin and adiponectin.
Further research is clearly required to fully assess the interactions between body composition and chemotherapy toxicity and effectiveness(Reference James, Wootton and Jackson97). Techniques such as dual-energy X-ray absorptiometry, bioelectrical impedance, computerised tomography scan software and MRI analysis provide a clearer picture of body composition and may have a role in the management and treatment planning of obese patients in the future(Reference Renehan, Harvie and Cutress88). However, clearly the risk-benefits and cost-effectiveness of such approaches will need to be addressed as currently it is not a routine practice for all early breast cancer patients to undergo cross-sectional imaging for staging purposes.
Health behaviour
Finally, it cannot be excluded that obesity or body fatness are surrogate markers for other health behaviours in addition to dietary ones. The WCRF continuous update project on diet, nutrition, physical activity and breast cancer survivors additionally investigated the impact of physical activity both before and after a diagnosis of breast cancer on long-term survival. They concluded that there was limited but consistent evidence that physical activity prior to diagnosis was associated with a reduction in both all-cause and breast cancer mortality(7). The same conclusion was reached regarding physical activity after breast cancer diagnosis. Similar results were reported by the After Breast Cancer Pooling Project, which reported a 27 % significant decreased risk of mortality and a 25 % reduction in breast cancer mortality associated with engagement in at least 10 metabolic equivalent per task hours per week compared with <10 metabolic equivalent per task hours per week(Reference Beasley, Kwan and Chen98). The WCRF has therefore concluded that there is sufficient evidence to recommend that early breast cancer patients follow the WCRF cancer prevention recommendations, which include being physically active in addition to eating a healthy diet and maintaining a healthy weight. However, more evidence is needed in order to make specific recommendations on lifestyle modifications for breast cancer survivors. Prospective studies to evaluate whether weight loss and lifestyle interventions will improve breast cancer outcomes are now in progress.
Conclusions
The obesity epidemic in both developed and developing countries poses challenges in terms of both increasing cancer incidence and specific management considerations. Obesity at diagnosis is associated with reduced breast cancer-specific and overall survival. Research is required to fully understand the biological mechanisms behind this as well as the practical implications of obesity on treatment effectiveness and tolerance (Fig. 2). Work is also required to fully elucidate body composition patterns in order to define the true nature of the risk factor presented by obesity in early breast cancer. With greater understanding of the link between obesity and breast cancer outcome, specific treatment strategies, including lifestyle interventions, can be developed to improve the prognosis of this patient group.
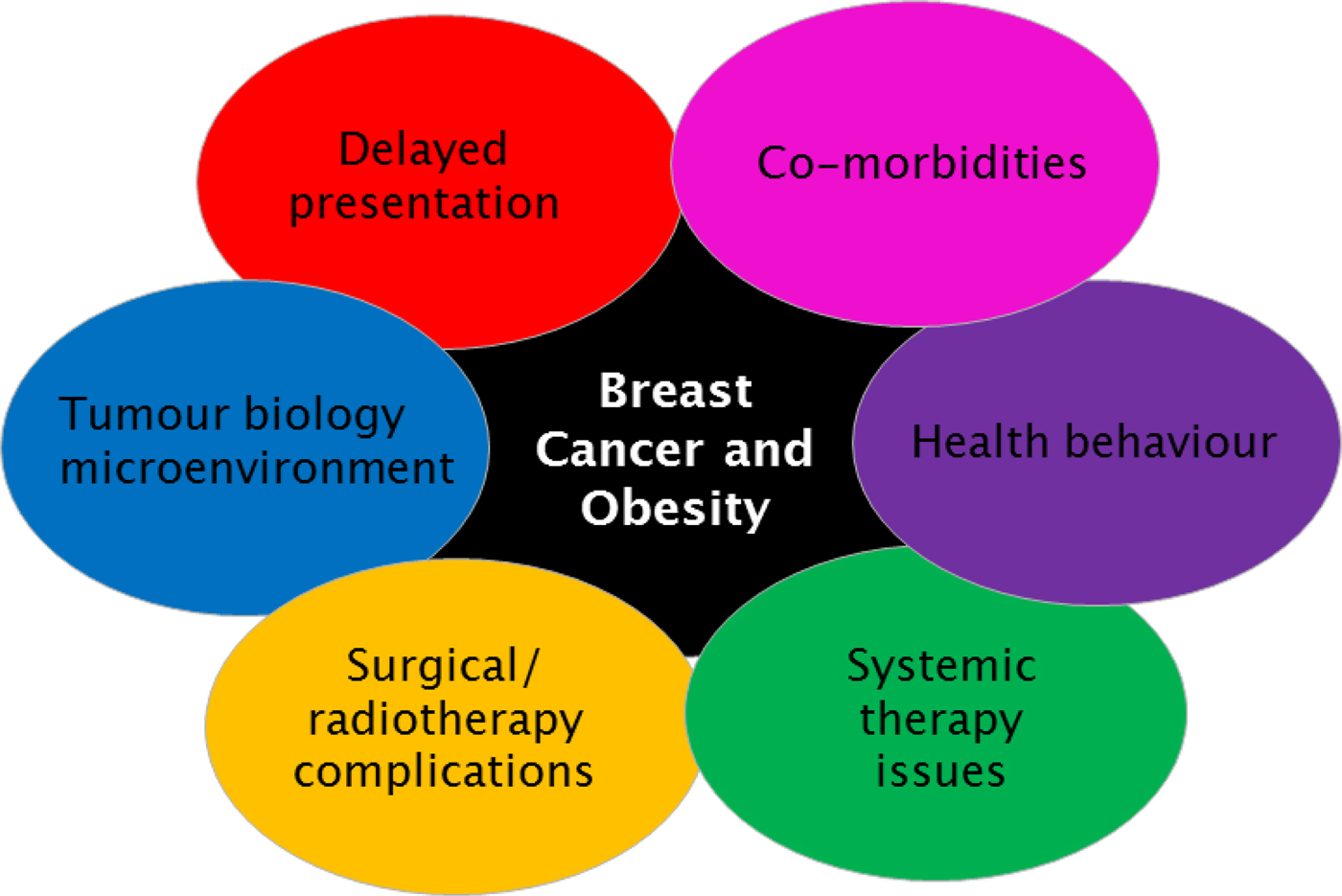
Fig. 2. (Colour online) Interacting factors in obese patient with early breast cancer, which may adversely affect prognosis.
Acknowledgements
The authors thank the NIHR Southampton Biomedical Research Centre in Nutrition. A body composition analyser (SECA mBCA 515) has been provided to the NIHR Southampton Biomedical Research Centre in Nutrition by SECA.
Financial Support
E. R. C. is supported by Cancer Research UK and has received honoraria from the WCRF. R. I. C. is grateful for support from the Biomedical Research Centre-Nutrition University Hospital Southampton. These funders have had no role in the design, analysis or writing of this paper.
Conflicts of Interest
None.
Authorship
This paper was jointly conceived and written by the three authors. All authors have approved the submitted manuscript.







