Mandatory fortification of staple foods with folic acid is a feasible policy option for reducing the prevalence of neural tube defects (NTD). The objective of this study was to estimate the impact of addition of folic acid to bread or flour on reducing the risk of occurrence of NTD-affected pregnancies and on the possible risk of masking (undiagnosed) vitamin B12 deficiency in older adults. Analyses were based on data from the National Adult Nutrition Survey (2008–2010) (www.iuna.net). Food composition data were updated to reflect current levels of folic acid in breads and fat spreads and estimates of folic acid intakes factored in an overage of 25 % for (voluntarily) fortified foods and supplements. Reduction in risk of occurrence of NTD-affected pregnancies was estimated from the increase in average daily folic acid intake in women aged 18–50 years(Reference Daly, Kirke and Molloy1)(Reference Daly, Mills and Molloy2). Possible risk of masking anaemia associated with (undiagnosed) vitamin B12 deficiency in adults >50 years was assessed from the probability of exceeding the Tolerable Upper Intake Level (UL) for folic acid (1,000μg), and the magnitude of any possible excess. Usual intakes of folic acid were calculated via the NCI-method(Reference Tooze, Kipnis and Buckman3) using SAS© Enterprise Guide. DaDiet© Version 15.05 was used to investigate simulation scenarios for addition of folic acid to: 1) all breads at a level of 120μg/100 g (as consumed), 2) all breads at a level of 225μg/100 g, 3) all wheat flour at a level of 225μg/100 g, 4) scenario 3 but excluding folic acid intake from voluntarily fortified foods, 5) scenario 3 but excluding folic acid intake from supplements and 6) scenario 3 but excluding folic acid intake from voluntarily fortified foods and supplements.
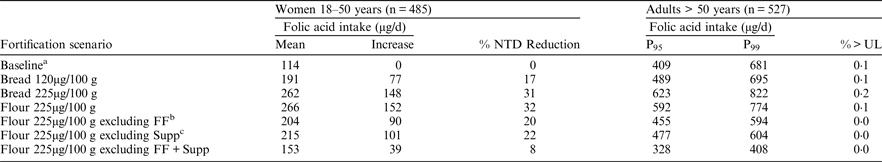
aBaseline represents current intakes of folic acid from voluntarily fortified foods and supplements, including an additional 25 % as overage. bFF = Fortified foods, cSupp = Supplements
These fortification scenarios would reduce the risk of NTD-affected pregnancies by 8–32 %, corresponding to an increase of 39–152μg in the mean daily folic acid intake of women of childbearing age. The risk of masking anaemia associated with (undiagnosed) vitamin B12 deficiency in older adults would be negligible as the probability of exceeding the UL for folic acid, even by a small amount, is very low (⩽0·2 %). P95 and P99 intakes of folic acid did not exceed 623μg/d or 822μg/d, respectively, for any scenario. These levels of addition of folic acid to bread and flour would allow safe consumption of folic acid at current levels from other fortified foods and supplements. Currently foods that are voluntarily fortified with folic acid reduce the risk of occurrence of NTD-affected pregnancies by about 11–14 %.
The study was commissioned by the Food Safety Authority of Ireland. The National Adult Nutrition Survey was funded by the Irish Department of Agriculture, Food and the Marine.


