Atherosclerosis-related cardiovascular disease (CVD) represents a great burden to human health. Inflammation and oxidative stress have both been implicated in playing important roles in the development of atherosclerosis and in addition, diet also plays a significant role in modulating the disease process. In this study, the role played by two dietary components, vitamin D and selenium (Se) both individually and together, have been investigated in a cellular model of atherosclerosis. Vitamin D is a known modulator of immune function in addition to its classical effects on calcium and bone homeostasis (Reference Kamen and Tangpricha1) whereas Se, mainly through its incorporation into selenoproteins, plays an important anti-oxidant role (Reference Shrimali, Irons and Carlson2). Each of these micronutrients, which are known modulators of immune function and oxidative stress respectively, therefore potentially play a role in influencing atherosclerotic development.
We hypothesised that in both immune cells and endothelial cells, vitamin D could potentiate the function of Se and conversely, that Se promoted the action of vitamin D and that these interactions would influence atherosclerosis. Potential interactions between both nutrients were assessed by measuring the effect of each nutrient on the expression of genes (by qPCR) known to be involved in the action of the other nutrient (i.e. cystathionine beta-synthase (CBS) for Se and 25-hydroxyvitamin D3 1-alpha-hydroxylase (CYP27B1) for vitamin D). CBS is the enzyme that converts homocysteine to cystathionine but which is also involved in incorporating Se into selenoproteins, and CYP27B1 converts 25-hydroxyvitamin D3 (25(OH)D3) to its active 1,25(OH)2D3 form. In addition, the effect of vitamin D on Se function was assessed by assaying its effect on glutathione peroxide activity (GPx) in immune cells. Lastly, the effect of both micronutrients on basal and pro-inflammatory-induced adhesion of monocytes to endothelial cells was assayed by an in vitro adhesion assay. Human monocytes (U937 cells) were cultured with or without Se and/or 25(OH)D3 and the expression of CYP27B1 and CBS quantified. Additionally, GPx activity was measured in cell lysates. For the monocyte-endothelial adhesion assay, HUVECs were stimulated with and without TNF-α and adhesion of U937 cells quantified by fluorescence.
Results showed that Se augmented the expression of CYP27B1 (Fig A; p < 0.001) and also that CBS expression was upregulated by vitamin D (p < 0.01; data not shown). GPx activity at suboptimal Se concentrations was shown to be stimulated by 25(OH)D3 to levels achieved with optimal Se (Fig B; p < 0.01). Se was shown to attenuate both basal and cytokine-stimulated adhesion of monocytes to endothelial cells and that the presence of vitamin D further reduced basal adhesion (Fig C; p < 0.05). Data are represented as means ± SEM (n = 3). Student's t-test was used to determine differences between treatments, *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant.
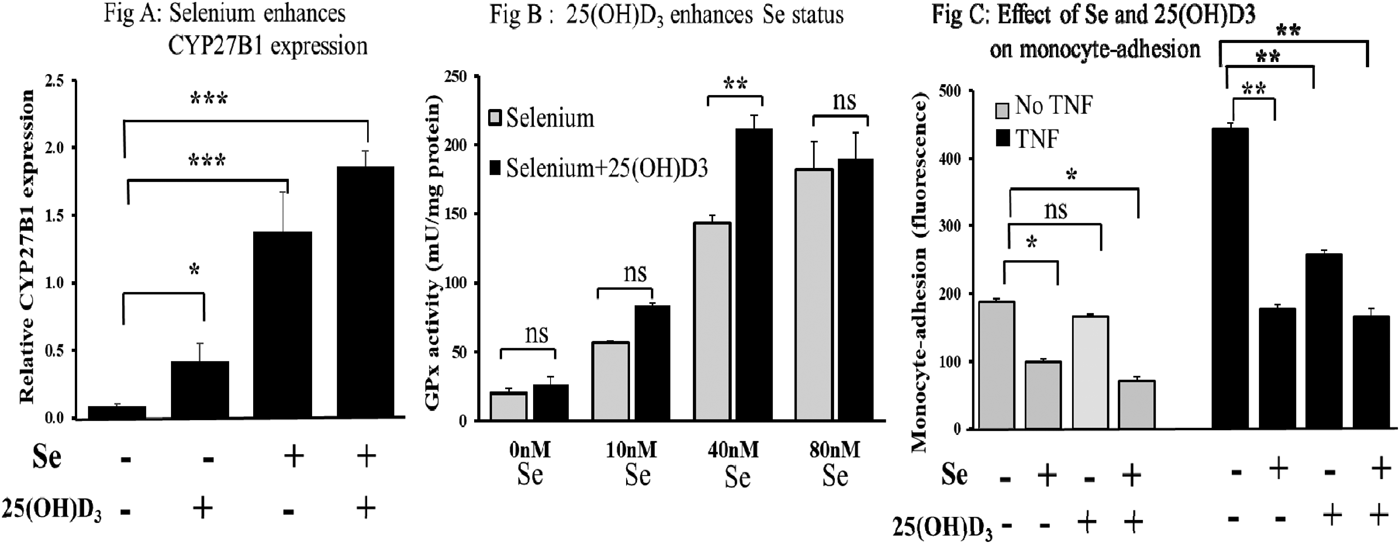
In conclusion, the presence of Se may act to enhance localised levels of active vitamin D and conversely, vitamin D may act to enhance Se status. This study therefore provides the first evidence for a potential synergistic interaction of vitamin D and Se which together may enhance both immune and endothelial function and slow atherosclerotic development.


