Quinoa (Chenopodium quinoa Willd.) is a traditional Andean seed crop with a favourable phytochemical composition and a high quality fatty acid profile(Reference Filho, Pirozi and Borges1, Reference Simnadis, Tapsell and Beck2). While the anti-inflammatory, antioxidant and lipid-lowering potential of such bioactive components are well established(Reference De Carvalho, Ovidio and Padovan3); the potential of quinoa specifically to exert such effects in vivo has not yet been fully elucidated in controlled human trials. The present study therefore investigated the effect of consuming quinoa-enriched biscuits on markers of CVD risk over 4-weeks in free-living older adults.
A randomised double blind crossover trial was conducted in which consenting healthy adults aged 50–75 years (n 40) consumed 2 × 15 g quinoa-enriched biscuits (60 g quinoa flour/100 g) or control iso-energetic biscuits (made using wheat flour) daily for 28 consecutive days (4-weeks), in addition to their normal diet. Following a 6-week washout, participants consumed the alternate biscuit for a final 4 weeks. Anthropometrics and fasted blood samples were obtained before and after intervention, and samples were analysed for lipid profiles on the iLab 650 Clinical Chemistry auto-analyser and for total polyunsaturated fatty acid (PUFA) concentrations by GC-MS(Reference Strain, Davidson and Bonham4).
At the beginning of the trial, mean (SD) total cholesterol concentrations were 6·02 (1·22) mmol/L (range 3·7–9·2 mmol/L); 33 subjects (82·5%) had high cholesterol (>5 mmol/L). No participants were lost to follow-up and there were no changes in habitual dietary intakes or levels of physical activity between each 4-week intervention period.
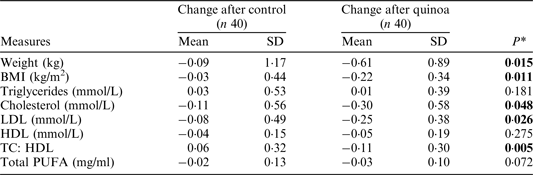
BMI, body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein; PUFA, polyunsaturated fatty acid. *Response to treatment (after–before) were significantly different between groups, P < 0·05 (1-tailed paired t-test).
A significantly greater decrease in body weight, BMI, total and LDL cholesterol concentrations, and TC:HDL ratio were apparent following consumption of the quinoa-enriched biscuits compared to the control biscuits. No significant differences in the change in triglycerides, HDL cholesterol, total PUFA or CRP concentrations were observed between treatment groups.
Consumption of novel quinoa-enriched biscuits produced favourable changes in body weight, BMI and circulating cholesterol concentrations, which may contribute to lowered CVD risk in free-living older adults. Changes in PUFA status alone cannot explain these findings, and therefore future research is required to elucidate quinoa's potential cardio-protective effects.
The trial was funded by CONICYT Chile/UK (ref: R15F10005). Ethical approval was obtained from the Ulster University Research Ethics Committee (REC/16/0106). The study was conducted according to the guidelines laid down in the Declaration of Helsinki and the trial was registered at www.clinicaltrials.gov (NCT03291548).


