Article contents
Bark, a suitable biosorbent for the removal of uranium fromwastewater – From laboratory to industry
Published online by Cambridge University Press: 16 December 2011
Abstract
This paper shows that natural materials such as barks can successfully replace syntheticresins for industrial purposes. Evaluated in batch conditions, biosorption of uranium onsuitably prepared Douglas fir barks took place in less than 10 min and appeared to beoptimum at pH>4. The biosorption process of uranium (uranyl formUO\hbox{$_{\mathrm{\mathbf{2}}}^{\mathrm{\mathbf{2+}}}$}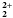 2+2)was characterized in the optimal physico-chemical conditions and could be mathematicallymodeled as a Langmuir isotherm. With a maximum uranium specific uptakeqmaxvalue of 1.16 meq.g-1 (138mgU.g-1) it was found that the sorption capability of Douglas fir barks wasat least five times higher for uranium than for other heavy metals such as lead.Adsorption of uranium contained in water leached from a former uranium mine was thenmonitored over a one-month period in a laboratory-scale chromatography column. Thefixation capacity remained fairly constant throughout the whole testing period. Waterradioactivity decreased from 1500 mBq.L-1 (0.12 mgU.L-1) to <5 mBq.L-1(0.4 μ gU.L-1) at the columnexit. This technology was successfully transferred and tested through a pilot projectunder industrial conditions with the support of AREVA NC.
2+2)was characterized in the optimal physico-chemical conditions and could be mathematicallymodeled as a Langmuir isotherm. With a maximum uranium specific uptakeqmaxvalue of 1.16 meq.g-1 (138mgU.g-1) it was found that the sorption capability of Douglas fir barks wasat least five times higher for uranium than for other heavy metals such as lead.Adsorption of uranium contained in water leached from a former uranium mine was thenmonitored over a one-month period in a laboratory-scale chromatography column. Thefixation capacity remained fairly constant throughout the whole testing period. Waterradioactivity decreased from 1500 mBq.L-1 (0.12 mgU.L-1) to <5 mBq.L-1(0.4 μ gU.L-1) at the columnexit. This technology was successfully transferred and tested through a pilot projectunder industrial conditions with the support of AREVA NC.
- Type
- Research Article
- Information
- Copyright
- © EDP Sciences, 2011
References
- 10
- Cited by


