The evidence base for prescribing a patient more than one antipsychotic drug at a time (antipsychotic polypharmacy) is limited and heterogeneous, and only supports the practice for individual patients who are severely ill and poorly respond to treatment. Reference Correll, Rummel-Kluge, Corves, Kane and Leucht1 Yet such prescriptions are common. Reference Harrington, Lelliott, Paton, Okocha, Duffett and Sensky2 The evidence for using clozapine alongside other antipsychotic medication for the treatment of schizophrenia is limited and contradictory, Reference Munro, Matthiasson, Osborne, Travis, Purcell and Cobb3-Reference Honer, Thornton, Chen, Chan, Wong and Bergmann5 but the practice appears to be common internationally Reference Pai, Laidlaw and Vella6 and may be modestly beneficial. Reference Taylor, Smith, Gee and Nielsen7 Although potentially the best medication for schizophrenia because of its efficacy, clozapine has been documented to interact with a number of drugs. Reference Sandson, Cozza, Armstrong, Eckermann, Fischer and Phillips8 Prescribing additional antipsychotics with clozapine may be associated with increased risk of agranulocytosis, Reference Godleski and Sernyak9 electrocardiogram (ECG) changes Reference Friedman, Ault and Powchik10 and long-term increased mortality. Reference Waddington, Youssef and Kinsella11 In an editorial which stimulated debate about additional antipsychotic prescribing in general, Reference Taylor12-Reference Lepping and Harborne14 Taylor stated that additional antipsychotic usage with clozapine is unlikely to be of value other than in the cases of cross-tapering when switching, rapid tranquillisation or specifically with the addition of aripiprazole. High-dose regimens, causing increased rates of dose-related adverse events including QT elongation, Reference Reilly, Ayis, Ferrier, Jones and Thomas15 can result from clozapine when used alongside other antipsychotics. Taylor Reference Taylor12 concludes with the hope that the audit procedures recommended by the National Institute for Health and Care Excellence (NICE) will be instrumental in reducing the extent of antipsychotic polypharmacy in psychiatric units in the UK.
Method
We performed an audit to identify how many patients were prescribed additional antipsychotic medication alongside clozapine, and to ascertain whether such patients were monitored appropriately if their prescriptions were in the high-dose range. The population was all those patients in hospital and in the community who had clozapine dispensed by our hospital’s pharmacy department.
The standards set for the audit were:
-
1 Where an additional antipsychotic was co-prescribed with clozapine, this was done in accordance with the local guideline, 16 which stated that it was acceptable that more than one antipsychotic was prescribed provided a partial response to clozapine had been documented.
-
2 Where high-dose antipsychotic treatment was prescribed, monitoring was performed in accordance with the local guideline, 17 which stated that monitoring of defined physical parameters should be carried out if additional antipsychotic prescribing caused the prescription to enter the high-dose range.
The definition of high dose in this audit was where the total daily dose of an antipsychotic exceeded the British National Formulary (BNF) maximum for that drug, or if more than one antipsychotic was used, the combined percentages of the BNF maximum dose for each drug exceeded 100%. The maximum dose for trifluoperazine was considered to be 30 mg.
All patients receiving clozapine from our pharmacy department during the audit had their case records accessed, and the following information collected. First, the dose of clozapine was recorded. If the patient was prescribed an additional antipsychotic, then the dose was recorded and the cumulative dose calculated as a percentage of BNF maximum dose. In relation to standard 1, evidence of prior partial response to clozapine before the introduction of a second antipsychotic was sought. Sufficient evidence was accepted as documentation of one or more of the following: rating scales showing evidence of improvement; accounts from the patient or carer of improvement in symptoms or general function; or clinician’s report of improvement.
For patients in the high-dose range, verification was sought that appropriate monitoring was carried out in the 3 months prior to retrieval. This consisted of urea and electrolytes, liver function tests, blood pressure, ECG, and temperature (the guideline 17 changed after the first cycle of the audit, and this requirement was removed for the subsequent cycles). Blood results were accessed first as these existed in electronic form. If the required blood tests had been performed, then the hospital records were consulted for the remaining information. Where there was no information in the records, the patient’s general practitioner (GP) was asked to consult their records to see whether the tests had been performed. The responsibility for monitoring in-patients rested in secondary care. Responsibility for monitoring out-patients rested in primary care, but psychiatrists were expected to alert GPs if the patient entered the high-dose range.
The first cycle of the audit was carried out during January-June 2008. The second cycle of the audit was carried out during August-November 2009. As the protocol for high-dose monitoring had changed between the first and the second cycle, removing the requirement for temperature monitoring, a third audit cycle was carried out during August-September 2010.
Table 1 Attainment of standards by year

| Audit cycle | Patients prescribed clozapine n |
Patients prescribed clozapine and additional antipsychotics n (%) |
Standard 1 n (%) |
Patients on high-dose antipsychotics n |
Standard 2 n (%) |
|---|---|---|---|---|---|
| I: 2008 | 169 | 45 (26) | 45 (100) | 10 | 1 (10) |
| II: 2009 | 185 | 51 (28) | 49 (96) | 19 | 4Footnote a (21) |
| III: 2010 | 193 | 60 (31) | 58 (97) | 18 | 5Footnote b (28) |
a. This figure is estimated because we were unable to gather data on two patients from general practitioner records; the true figure is between 3 and 5 (16-26%).
b. If patients on ‘as required’ medication who were assumed to be high-dose are removed, the figure falls to 4 out of 16 (25%).
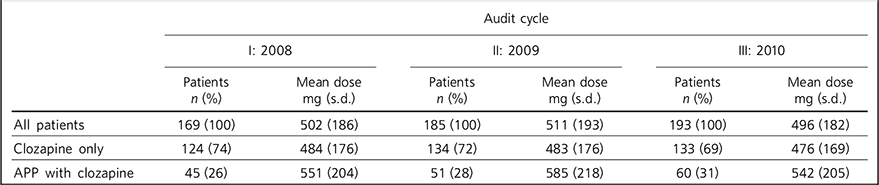
Table 2 Doses of clozapine by patient group
| Audit cycle | ||||||
|---|---|---|---|---|---|---|
| I: 2008 | II: 2009 | III: 2010 | ||||
| Patients n (%) |
Mean dose mg (s.d.) |
Patients n (%) |
Mean dose mg (s.d.) |
Patients n (%) |
Mean dose mg (s.d.) |
|
| All patients | 169 (100) | 502 (186) | 185 (100) | 511 (193) | 193 (100) | 496 (182) |
| Clozapine only | 124 (74) | 484 (176) | 134 (72) | 483 (176) | 133 (69) | 476 (169) |
| APP with clozapine | 45 (26) | 551 (204) | 51 (28) | 585 (218) | 60 (31) | 542 (205) |
APP, antipsychotic polypharmacy
Between each cycle, the results were disseminated as follows, emphasising the low rate of attainment of the second standard:
-
1 each general adult, old age, adolescent, forensic, rehabilitation, and learning disability consultant psychiatrist received a summary of the results (and between the second and third cycles they also received specific feedback for their named patients);
-
2 the results of the audit were presented as part of an ordinary meeting of the area educational programme, to which all grades of medical staff were invited;
-
3 a report was submitted to the local clinical governance group.
Results
The percentage of patients on clozapine who were prescribed additional antipsychotics (including cross-tapering when switching, rapid tranquillisation, and specific augmentation strategies) was around 30%. The attainment of standard 1 was high, consistently close to 100%. The attainment of standard 2 was low, initially 10%, and only rose to 28% in the third cycle (Table 1).
The differences in clozapine dose between patients taking clozapine only and patients taking clozapine with additional antipsychotics are small (Table 2). Patients on antipsychotic polypharmacy were on higher doses, but not near-maximum doses.
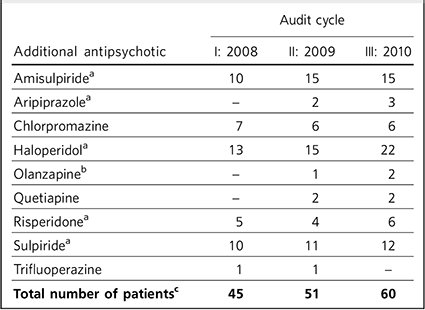
Increasing proportions of patients on clozapine were prescribed additional antipsychotics over the course of the three audit cycles. The range of additional antipsychotic agents is seen in Table 3. Combinations of clozapine with up to two additional antipsychotic agents were used.
Table 3 Numbers of patients prescribed additional antipsychotics alongside clozapine
| Audit cycle | |||
|---|---|---|---|
| Additional antipsychotic | I: 2008 | II: 2009 | III: 2010 |
| AmisulpirideFootnote a | 10 | 15 | 15 |
| AripiprazoleFootnote a | - | 2 | 3 |
| Chlorpromazine | 7 | 6 | 6 |
| HaloperidolFootnote a | 13 | 15 | 22 |
| OlanzapineFootnote b | - | 1 | 2 |
| Quetiapine | - | 2 | 2 |
| RisperidoneFootnote a | 5 | 4 | 6 |
| SulpirideFootnote a | 10 | 11 | 12 |
| Trifluoperazine | 1 | 1 | - |
| Total number of patientsFootnote c | 45 | 51 | 60 |
a. Evidence for utility in combination with clozapine. Reference Taylor, Paton and Kapur18
b. Poorly supported by evidence and not recommended. Reference Taylor, Paton and Kapur18
c. The total number of patients is smaller than the sum of the figures in the column because some patients were prescribed clozapine with not just one, but two additional antipsychotic medications
Not all additional antipsychotic medication was prescribed on a regular basis. In the three cycles of the audit, respectively 11%, 8% and 11% of patients receiving clozapine were also prescribed additional antipsychotics on an ‘as required’ (p.r.n.) basis for symptom control. Such patients made up a substantial minority of the clozapine antipsychotic polypharmacy prescriptions (which were 26%, 28% and 31% respectively for the three cycles).
Prescription of additional antipsychotic p.r.n. medication could potentially bring patients into the high-dose range. Where this occurred for in-patients, antipsychotic use was calculated from dispensing records. Where this occurred for out-patients, it was estimated. In the first cycle, 18 patients (9 out-patients and 9 in-patients) of the group of 45 patients on clozapine antipsychotic polypharmacy were taking p.r.n. antipsychotics alongside clozapine. From ward dispensing records, it was calculated that none of the in-patients were in the high-dose range. No such records existed for out-patients, so it was assumed that, like the in-patients, none would enter the high-dose range. However, this may not have been the case, and the low level of achievement of the secondary standard in the first cycle may actually have been even lower. This potential source of error could not exist in the second cycle because there were no such out-patients. In the third cycle the two out-patients in the situation where antipsychotic polypharmacy medication could drive them into the high-dose range were assumed to be high-dose, because they were already on maximum doses of regular antipsychotic medication and a single instance of p.r.n. medication would have taken them into the high-dose range. If these two patients were removed from the analysis, then adherence to the secondary standard in the third cycle would fall further to 25%, because one of the two was in the group of patients receiving high-dose monitoring.
Patient refusal would have been considered a justifiable reason for the absence of high-dose investigations where these were indicated. However, this was not recorded as a reason for the absence of high-dose investigations in any of the notes of the patients in the high-dose groups in the study period.
Discussion
Overall, the attainment of standard 1 was high. The same two patients caused standard 1 to be failed in both the second and third cycle.
It is known that antipsychotic polypharmacy can lead to high-dose prescribing. Reference Tungaraza, Gupta, Jones, Poole and Slegg19 However, it is disturbing that so few of the patients who met high-dose criteria received appropriate monitoring (standard 2). There may have been confusion among prescribers between high-dose monitoring and clozapine physical state monitoring. High-dose monitoring and clozapine physical state monitoring are not equivalent in our area and are unlikely to be uniform in other regions. Neither set of requirements subsumes the other; both should be conducted on patients on clozapine antipsychotic polypharmacy who are in the high-dose range. Prescribers should be alert to this when combining even moderately high doses of clozapine with other antipsychotic medications.
In this audit, however, this explanation seems less likely because consultants were reminded of this requirement before subsequent audit cycles on two occasions, and the proportion of patients satisfying standard 2 still remained low. It seems more probable that patients have moved into the high-dose range without prescribers noticing or that prescribers are simply ignoring the requirement for high-dose monitoring. In our area, the hospital pharmacy dispensed clozapine, whereas other antipsychotic drugs are dispensed by the community pharmacists, therefore only prescribers were in a position to notice and instigate monitoring when patients were pushed into the high-dose range.
A number of studies have shown poor adherence to standards in monitoring antipsychotic medication. Reference Odelola and Ranceva13 Although prescribers may feel that they have no option but to use high-dose antipsychotic polypharmacy in certain situations, such as with complex and dangerous patients, the question remains as to why they do not monitor patients appropriately. Prescribers may have doubts about the relevance of monitoring or feel that monitoring will not mitigate the risks of high-dose prescriptions. Patients may also refuse to have appropriate high-dose investigations, which would be a justifiable reason for the absence of high-dose monitoring in individual cases, but this was not documented as a reason for any patient’s lack of appropriate monitoring in our audit. Indeed, it would be surprising if many of these patients were not adherent to high-dose investigations while remaining willing to comply with regular routine clozapine investigations - which was the case, or else their clozapine would not have been dispensed.
The differences in clozapine doses seen in Table 2 between patients taking only clozapine and patients with clozapine antipsychotic polypharmacy are small. The doses of clozapine in the polypharmacy groups in each cycle are considerably less than the maximum dose; therefore not all additional antipsychotic prescribing was due to incomplete response to clozapine at maximum dose. It follows that patients may have been prescribed additional antipsychotics for other reasons. Higher doses of clozapine can be poorly tolerated because of side-effects such as fitting, sialorrhoea or sedation. In these cases additional antipsychotic medication may have been prescribed to improve symptom reduction without producing such phenomena.
Not all additional antipsychotics were given regularly as an augmentation strategy. Clozapine cannot be taken ‘as required’ for symptom control. If there are ‘breakthrough symptoms’ and the prescriber chooses to act, then they have a choice of increasing the regular prescription of clozapine or adding another antipsychotic on a short-term basis. However, there is a lack of evidence for either option, and additionally ‘short-term’ prescriptions of a second antipsychotic may inadvertently become long-standing. Some of the out-patient prescribing of ‘as required’ additional antipsychotic medication appears to have been open-ended, and left to the discretion of the patient.
As there are no standards in our region for the use of clozapine with psychotropic drugs other than antipsychotics, it was not possible to audit such practices. However, in some instances the co-administration of these compounds may be more relevant than the co-administration of antipsychotics, for example, the use of some selective serotonin reuptake inhibitors with near-maximum doses of clozapine may cause raised plasma clozapine levels equivalent to high-dose prescribing, or the prescription of certain mood stabilisers which increase the risk of agranulocytosis.
Antipsychotic polypharmacy with clozapine may be justifiable for individual patients. Reference Taylor12 The evidence base for the necessity and utility of monitoring is weak. However, psychiatrists need to bear in mind that their practices may result in high-dose prescriptions, which current guidelines state require monitoring. In our audit, consecutive annual feedback to individual prescribers resulted only in a modest improvement in high-dose monitoring. As work from other areas in the UK suggests that antipsychotic polypharmacy use leads to high-dose prescriptions, Reference Tungaraza, Gupta, Jones, Poole and Slegg19 lack of appropriate monitoring of high-dose clozapine antipsychotic polypharmacy may be a widespread problem.






eLetters
No eLetters have been published for this article.