Introduction
Patent ductus arteriosus stenting has evolved as an alternative strategy to a Blalock–Taussig–Thomas shunt in patients with duct-dependent pulmonary circulation. Reference Glatz, Petit and Goldstein1,Reference Bentham, Zava and Harrison2 However, ductal stenting in duct-dependent pulmonary circulation is more challenging, because of its diverse morphology and different approaches may be necessary for successful stenting. Reference Alwi3,Reference Boshoff, Michel-Behnke, Schranz and Gewillig4 These ductuses tend to originate from the arch of the aorta and have more vertical and tortuous course. Reference Hinton and Michelfelder5,Reference Abrams and Kevin6,Reference Santos, Moll, Drumond, Araujo, Romao and Reis7 It often causes branch pulmonary artery stenosis of varying degrees at the site insertion. Reference Elzenga and Gittenberger-de Groot8 Hence, a detailed delineation of ductus arteriosus morphology and its relationship to the aorta and pulmonary artery is crucial, as it affects the feasibility and technical approach of the procedure. Echocardiogram may provide some information on the ductus morphology. However, complete delineation is difficult in most cases, especially in those with a tortuous course. In our centre, initially, most patients with duct-dependent pulmonary circulation underwent cardiac catheterisation with anticipation of ductal stenting. Reference Alwi3 Although this may be beneficial in suitable patients, this approach may subject unsuitable candidates to unnecessary invasive procedure and its associated complications. Furthermore, angiographic evaluation of ductus arteriosus is sometimes difficult due to the overlapping structures, despite multiple angiographic angulations. This may obscure the presence of proximal branch pulmonary artery stenosis and tend to underestimate the length of the ductus arteriosus.
Since the beginning of 2000s, there has been an increasing trend in utilising CT as a diagnostic tool in CHD to assess extravascular structures. Reference Gherardi, Iball, Darby and Thomson9 At the same time, ductal stenting was gaining a wider acceptance and expanded to more complex ductal anatomy. With the availability of dual-source CT in 2007, we performed CT in duct-dependent pulmonary lesions in those with tortuous ducts seen on echocardiogram for better delineation and to provide a 3-dimensional demonstration of ductus arteriosus morphology.
From January 2013 to December 2015, we prospectively performed CT to study the ductus arteriosus and pulmonary anatomy in all patients with duct-dependent pulmonary circulation prior to any intervention. The objectives of this study were as follows:
-
1. To assess the morphology of ductus arteriosus (site of origin, insertion, tortuosity, and presence of branch pulmonary artery stenosis) in those with duct-dependent pulmonary circulation.
-
2. To study the ductus arteriosus pattern in different types of ventricular morphology (univentricular, biventricular, and pulmonary atresia intact ventricular septum).
Material and methods
This is a prospective observational study conducted at National Heart Institute, Kuala Lumpur, Malaysia from January 2013 to December 2015. Ethics committee approval was obtained for the study, and parental consent was taken.
Those with duct-dependent pulmonary circulation aged 6 months and below, without any previous intervention were included in the study. All patients underwent echocardiography to establish the cardiac diagnosis. CT was performed in all to study the ductus arteriosus morphology.
Procedure
The CT was performed as a clinical research test using a dual-source 64-slice CT scanner (Siemens SOMATOM Definition, Germany) under light sedation (chloral hydrate at 50 mg/kg; IV midazolam at 0.5 mg/kg was added when necessary). The constrast was injected via a peripheral vein, using a double head injector. A total of 1–2 ml/kg of contrast was used. The image was acquired using a standard non-echocardiogram gated protocol. The CT images were analysed and reconstructed by a single assessor (HAL) using maximum intensity projection, multi-planar reformatting, and volume-rendered techniques to delineate the following features: aortic arch sidedness (right or left aortic arch), ductus arteriosus morphology, pulmonary artery anatomy, and the surrounding structures including the airway.
Patent ductus arteriosus morphology classification and definitions
The assessment of ductus arteriosus morphology included its site of origin, insertion, and tortuosity. The ductal origin was used as a key feature to classify the patent ductus arteriosus. We classified the ductus arteriosus as type I, II, or III based on its site of origin: type I – from descending aorta (distal to left subclavian artery in a left aortic arch), type II – from the aortic arch, and type III – from the subclavian artery. From our observation, a small number of type II ductus has a takeoff more proximally (close to the innominate artery), and they were usually reversely orientated. Reference Alwi3 Therefore, we further subclassify type II into IIa and IIb depending on the ductal origin from the distal aortic arch (i.e. between the takeoff of the left common carotid and left subclavian artery in the left aortic arch) or proximal aortic arch (i.e. from the origin of the innominate artery to the left common carotid artery in a left aortic arch), respectively (Figs 1b and 1c). The presence of bilateral ductus was also sought (Fig 1e and f). Tortuous ductus arteriosus was defined as ductus with multiple acute bends (i.e. more than a single bend).

Figure 1. Volume-rendered technique images showing different site of patent ductus arteriosus origin: (a) Ductus arteriosus arises from the descending aorta, type I, ( b ) from distal aortic arch, type IIa, ( c ) from proximal arch, type IIb, ( d ) from the base of innominate artery, viewing from posteriorly, type III. The dotted line proximal to left common carotid artery in the left aortic arch, demarcating proximal and distal arch, ( e and f ) bilateral ductus arteriosus with discontinuous pulmonary arteries: ( e ): 15 days old, pulmonary atresia ventricular septal defect with left aortic arch; right duct from distal arch to right pulmonary artery and left duct from left subclavian artery to left pulmonary artery. ( f ): 25 days old, unbalance atrioventricular septal defect pulmonary atresia with right aortic arch. Right duct from distal arch to the right pulmonary artery and left duct from left subclavian artery to left pulmonary artery.
The pulmonary artery anatomy was assessed for its size, confluence, and the presence of branch pulmonary artery stenosis. Branch pulmonary artery stenosis was defined as the narrowest diameter of 50% or more from the distal pulmonary artery (before the takeoff of the upper branch). The anatomy of the branch pulmonary artery was classified into normal, stenosed, discontinuous, or absent. To study the pattern of ductus arteriosus morphology in different ventricular morphology, the cardiac lesions were assigned to either pulmonary atresia intact ventricular septum or other duct-dependent pulmonary circulation lesions. In other duct-dependent pulmonary circulation lesions group, ventricular morphology was classified as either biventricular or single ventricular lesion based on the direction of surgical treatment (i.e. biventricular or single ventricular repair pathway) as assessed on the echocardiogram.
To complete the CT analysis, an assessment of cardiac anatomy was also routinely performed using a segmental analysis approach in all cases. During this study period, CT could not be performed in two patients with duct-dependent pulmonary circulation due to difficult venous access and CT machine breakdown, respectively, and one patient was excluded from the study due to poor image quality caused by movement artefacts. Any CT-related complications and scan time were recorded, and the estimated radiation dose was calculated. Initial palliative procedures performed were noted. The estimated radiation dose (mSv) for a dual-source CT was calculated using the formula dose length product (milliGray*cm) × 2 × conversion or k-factor. Reference Goo10 All CT images were reviewed together by the first three authors to decide on ductal morphology classification. In case of any disagreement, more senior consultants were consulted.
Statistical analysis
The patient’s demographic data are expressed as mean (standard deviation) or median (range). The ductus arteriosus morphology, pulmonary artery anatomy, and ductus morphology pattern in different ventricular morphology were expressed in frequency or percentage. The statistical analysis was performed using chi-square test or Fisher’s exact test, where appropriate. Data analysis was performed using SPSS version 27.0, and a p value of <0.05 was considered as significant.
Results
Demographic data
A total of 114 patients comprised of 64 (56.1%) males and 50 (43.9%) females successfully underwent CT angiography. Their median age and weight were 1.0 month (range 0.3–6 months) and 3.3 kg (range 2–6.9 kg), respectively. Seventy-nine (69%) patients were on prostaglandin infusion at the time of the CT study. Their cardiac diagnoses are summarised in Table 1. Twenty-two (19.3%) patients had pulmonary atresia intact ventricular septum. In diagnoses other than pulmonary atresia with intact ventricular septum, 53 (46.6%) patients had biventricular lesions and 39 (34.2%) had univentricular lesions. Tetralogy of Fallot with pulmonary atresia/pulmonary stenosis was the commonest cardiac lesion, comprising of 33 patients (28.9%), followed by pulmonary atresia intact ventricular septum (19.3%), tricuspid atresia/stenosis (11.4%), single ventricle with heterotaxy syndrome (8.7%), and congenitally corrected transposition of great arteries (6.1%).
Table 1. Cardiac diagnosis

AVSD: Atrioventricular septal defect; CCTGA: Congenitally corrected transposition of great arteries; DDPC: duct-dependent pulmonary circulation; DILV: Double inlet left ventricle; DORV: Double outlet right ventricle; PA/IVS: Pulmonary atresia intact ventricular septum; PS: Pulmonary stenosis; SV: Single ventricle; TGA: Transposition of great arteries; TOF: Tetralogy of Fallot; VSD: Ventricular septal defect.
Aortic arch sidedness
Twenty-five patients (21.9%) had a right aortic arch with a mirror image branching pattern, and 89 (78.1%) had a left aortic arch.
Morphology of ductus arteriosus
Only two patients had bilateral ductus arteriosus. One of them had dextrocardia, tricuspid atresia, ventricular septal defect, pulmonary atresia with a left aortic arch. The right ductus arteriosus was straight, arose at the takeoff of the right innominate artery (type III), supplying the right pulmonary artery and the left ductus arteriosus was tortuous, arose from the proximal aortic arch (type IIb) supplying the left pulmonary artery (Fig 1e). The second was a 15-day-old baby, who had pulmonary atresia ventricular septal defect with a right aortic arch. The right vertical ductus arteriosus was arising from the undersurface of the aortic arch (type IIa) supplying the right pulmonary artery, and the left ductus arteriosus was from the left subclavian artery supplying the left pulmonary artery (Fig 1f).
Each patent ductus arteriosus was considered as a unit. Therefore, a total of 116 ductus arteriosus were analysed for their origin, tortuosity, site of insertion to the pulmonary artery anatomy, and ductus arteriosus patterns in different ventricular morphology.
Ductus arteriosus origin
Thirteen ductus arteriosus (11.2 %) originated from the descending aorta (type I) of which 12/13 had pulmonary atresia with intact ventricular septum, 92 (79.3%) from the aortic arch (type II), and 11 (9.5%) from the subclavian or innominate artery (type III). Of the 92 type II patent ductus arteriosus, 71 (77.2%) were from the distal arch (type IIa) and 21 (22.8%) from the proximal arch (type IIb).
In patients with pulmonary atresia intact ventricular septum, 12/22 (54.5%) had type I, 10/12 (45.5%) had type II, and none has type III. While in patients with biventricular and single ventricular morphology the majority of ductus arteriosus originated from the aortic arch (type II), that is 84.9% (45/53) and 89.7% (35/39), respectively (Table 2). Of 22 patients with pulmonary atresia with intact ventricular septum, 15 had unipartite right ventricle and 7 had either bi-or tripartite right ventricle. 9/15 with unipartite ventricle (60%) had type II patent ductus arteriosus whilst only 1/9 (14.3%) with bi-or tripartite ventricle had type II patent ductus arteriosus (p value = 0.045).
Table 2. Ductus arteriosus morphology pattern in different ventricular anatomy

DDPC: duct-dependent pulmonary circulation; PAIVS: pulmonary atresia intact ventricular septum; PDA: patent ductus arteriosus.
Ductus arteriosus tortuosity
The majority of ductus arteriosus (77/116 ductus or 66.4%) were non-tortuous (Figs 2 a, b and c), whilst 39 ductus arteriosus (33.6%) were tortuous (Figs 2 c, d and e). Tortuous ductus arteriosus was found in 21/39 (53.8%) single ventricle, 13/53 (24.5%) in biventricular, and 4/22 (18.2%) in pulmonary atresia intact ventricular septum patients (Table 2)

Figure 2. Classification of ductus arteriosus tortuosity. The upper panel showing maximum intensity projection CT images of non-tortuous ductus arteriosus and lower panel volume-rendered technique CT images of tortuous ductus arteriosus.
Ductus arteriosus site of insertion and pulmonary artery anatomy
The ductus arteriosus was inserted into the left pulmonary artery in 62 (54.40%), the right pulmonary artery in 20 (17.85%), and the main pulmonary artery in 30 (26.78%). The branch pulmonary artery stenosis at the site of ductus arteriosus insertion was noted in 74 of 112 (66.0%) patients of which 6 (5.2%) had bilateral branch pulmonary artery stenosis (Fig 3). Unilateral branch pulmonary artery stenosis commonly (80.9%) occurs ipsilateral to the aortic arch, and usually, a small main pulmonary artery stump is present (Table 3). Further, analysing patients with bilateral branch pulmonary artery stenosis, five had type II ductus arteriosus and one had type III ductus arteriosus. The latter patient had a double outlet right ventricle with hypoplastic right ventricle. Discontinuous pulmonary artery was found in two patients, both with bilateral patent ductus arteriosus.

Figure 3. Site of insertion of ductus arteriosus and pulmonary artery anatomy. ( a and b ): Day 5 baby with pulmonary atresia intact ventricular septum, ductus arteriosus inserted into the main pulmonary artery. No branch pulmonary artery stenosis. (c and d ): 51-day-old patient with pulmonary atresia ventricular septal defect, ductus arteriosus from aortic arch inserted onto proximal left pulmonary artery, causing severe left pulmonary artery stenosis. Left pulmonary artery is hypoplastic. ( e and f ): 26 days old with right isomerism, unbalance atrioventricular septal defect. Long, straight duct inserted onto central pulmonary artery causing bilateral branch pulmonary artery stenosis. Absent main pulmonary artery stump.
Table 3. Pulmonary artery anatomy

LAA: Left aortic arch; LPA: Left pulmonary artery; MPA; Main pulmonary artery; PA: Pulmonary artery; PDA: Patent ductus arteriosus; RAA: right aortic arch.
In pulmonary atresia intact ventricular septum, the majority (77.3%) of ductus arteriosus were inserted into the main pulmonary artery and only 36.4% of patients had branch pulmonary artery stenosis [3/5 (60%) with unipartite right ventricle and 6/17 (35.3%)] in bi- or tripartite right ventricle]. On the contrary, in those with biventricular and single ventricle lesions, the majority of ductus arteriosus were inserted into the branch pulmonary arteries and had branch pulmonary artery stenosis (Table 4).
Table 4. Site of ductus arteriosus insertion and branch pulmonary artery stenosis in different cardiac diagnosis

AVSD: Atrioventricular septal defect; CCTGA: Congenitally corrected transposition of great arteries; DILV: Double inlet left ventricle; DORV: Double outlet right ventricle; MPA: Main pulmonary artery; PA: Pulmonary artery; PA/IVS: Pulmonary atresia intact ventricular septum; PS: Pulmonary stenosis; SV: Single ventricle; TGA: Transposition of great arteries; TOF: Tetralogy of Fallot; VSD: Ventricular septal defect.
* Exclude 1 patient with bilateral ductus arteriosus and disconnected pulmonary arteries.
Ductus arteriosus and airway compression
In our series, one patient with a large and tortuous type IIa ductus, in retrospect, had significant airway compression which worsened after ductal stenting requiring surgical removal of the stent and a right modified Blalock–Taussig shunt (Fig 4). This case was reported in our previous publication. Reference Rehman, Chaudhari, Latiff, Stumper and Alwi11
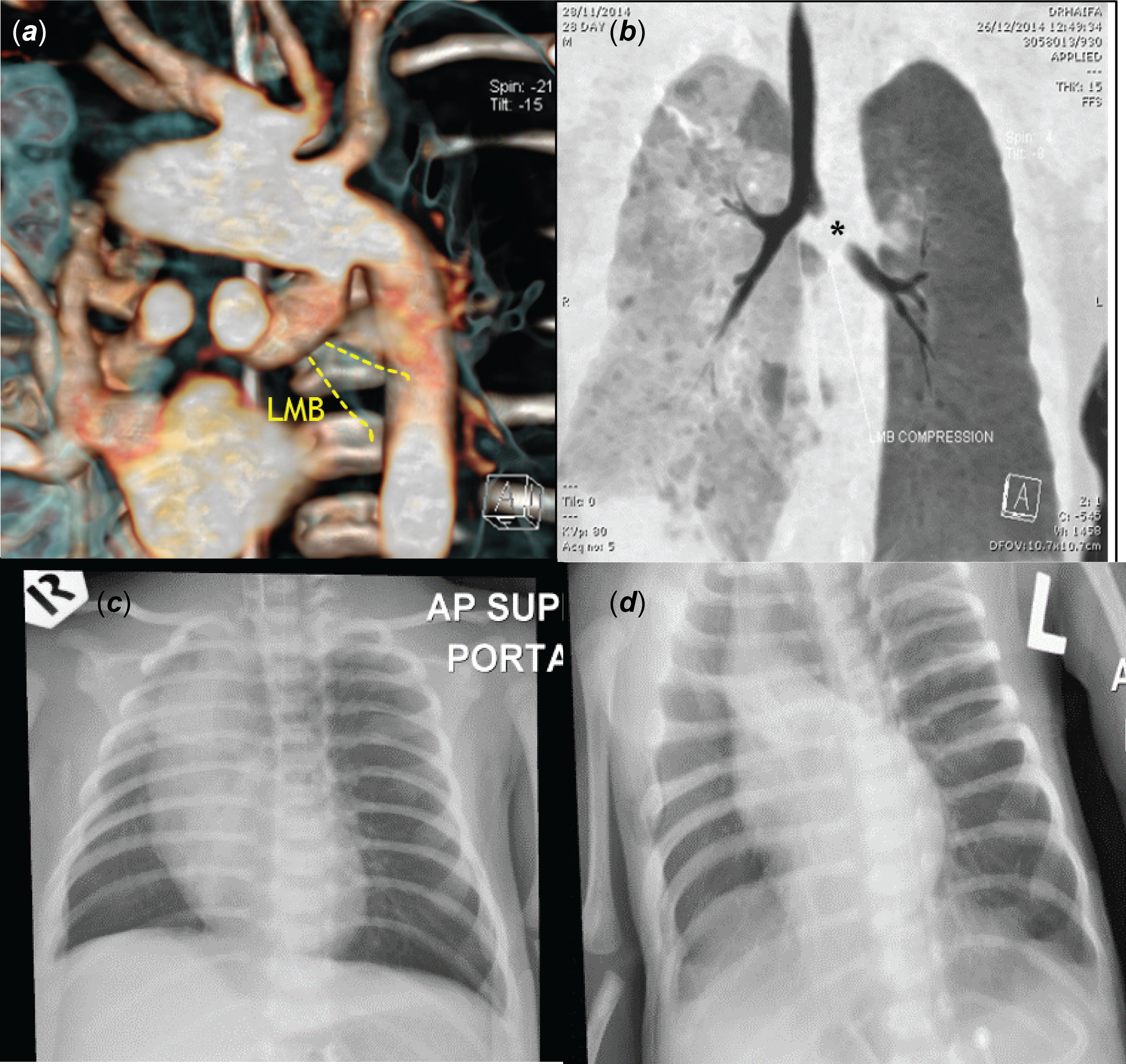
Figure 4. Airway compression by large ductus arteriosus in a patient with tricuspid atresia with ventricular septal defect, pulmonary atresia. ( a ) Volume-rendered technique image from posterior shows a large, tortuous duct arising from underneath the aortic arch. It forms a gentle curve proximally but turns acutely at midsegment, compressing the left main bronchus anteriorly, ( b ) CT bronchogram showed obstruction (*) of proximal left main bronchus, ( c ) pre-stenting chest X-ray in showed mild hyperinflation of left lung, ( d ) worsening of lung hyperinflation following ductal stenting.
CT-related complications
There were no CT-related complications. The mean scan time was 2.24 ± 0.082 seconds. Mean Dose Length Product and estimated radiation dose were 20.99 ± 13.57 milliGray*cm and 1.33 ± 0.095 millisievert, respectively.
Discussion
Study justification
Patent ductus arteriosus morphology has become a subject of interest with the increasing trend of ductal stenting in duct-dependent pulmonary circulation as an alternative option to the Blalock–Taussig–Thomas shunt since about a decade ago. From angiographic studies of ductal morphology prior to stenting, interventionists observed that ductal morphology in patients with pulmonary atresia with intact ventricular septum has a different characteristic compared to those with either biventricular or single ventricular lesions. Reference Alwi3 In the same era, CT scan had been frequently utilised to delineate small vascular structures in children with CHD as it is non-invasive. This study utilised non-invasive CT scan to study the spectrum of patent ductus arteriosus morphology in duct-dependent pulmonary circulation lesions.
Principal findings
Our study showed that the ductus arteriosus in duct-dependent pulmonary circulation has a wide morphological variation in its site of origin, tortuosity, propensity for branch pulmonary stenosis at site of insertion, with a distinct pattern between pulmonary atresia intact ventricular septum and other duct-dependent pulmonary circulation lesions. In pulmonary atresia intact ventricular septum, more than half (54.5%) of the patent ductus arteriosus arose from the descending aorta (type I), with the majority (81.8%) being non-tortuous and most (about 70%) inserted into the main pulmonary with no significant branch pulmonary artery stenosis. These findings confirm our previous observations and explain the higher success rate of ductal in this group of patients. Reference Alwi3,Reference Udink ten Cate, Sreeram, Hamza, Agha, Rosenthal and Qureshi12 Whilst majority (87.0%) of ductus arteriosus in other duct-dependent pulmonary circulation lesions arise from the arch of the aorta (type II), 37.0% were tortuous. More than half (53.8%) of patients with single ventricular lesion had tortuous ductus arteriosus. Interestingly, 60% of pulmonary atresia with intact ventricular septum with unipartite right ventricle had type II patent ductus arteriosus compared to only 14.3% in bi-or tripartite ventricle. The presence of type II patent ductus arteriosus in pulmonary atresia with intact ventricular septum patients may be the indicator for future single ventricular repair; however, this requires further study with a larger cohort of patient.
Type III and bilateral ductus arteriosus were uncommon (7.7% and 17.5%, respectively). Bilateral ductus arteriosus results from the interruption of the proximal sixth primitive aortic arch system, causing distal ductal origin of the pulmonary arteries. Previous studies have found that there is a considerable heterogeneity among congenitally malformed hearts associated with bilateral ductus arteriosus and the prevalence of bilateral ductus arteriosus is higher (25%) in the heterotaxy syndrome. Reference Formigari, Vairo, dezorzi, Santoro and Marino13,Reference Freedom, Pelech and Jeffrey14 In our series, one of 10 patients with heterotaxy syndrome had bilateral ductus arteriosus. The origin of the bilateral ductus arteriosus was consistent with the laterality of the aortic arch and the presence or absence of an aberrant subclavian artery. Reference Freedom, Pelech and Jeffrey14 In a left aortic arch, the left duct arises from the aortic arch and the right duct arise from the right subclavian artery and vice versa. About 66% of patients in our cohort had branch pulmonary artery stenosis at the site of ductus arteriosus insertion, the majority ipsilateral to the aortic arch. These findings concur with the previous retrospective review on specimens with pulmonary atresia and pulmonary stenosis by Elzenga et al. Reference Santos, Moll, Drumond, Araujo, Romao and Reis7 Branch pulmonary artery stenosis worsened with ductal restriction resulting in hypoplasia of the distal pulmonary artery and may become discontinuous when the ductus closes. Figure 3c and d showed tight stenosis of the left pulmonary artery at ductal insertion in 26- and 51-day-old neonates who presented late when the patent ductus arteriosus had become very restrictive. The marked discrepancy in the size of stenosed branch pulmonary artery is possibly due to preferential flow into the opposite branch, causing compromised perfusion and retarded growth to the affected branch pulmonary artery.
To our knowledge, this is the largest cohort of ductal morphology study in duct-dependent lesions. Our cohort involved a wide spectrum of complex cardiac anatomy, which compares well with the reported prevalence of critical congenital cyanotic heart lesions in this region. Reference Bah, Sapian and Alias15
In a recent angiographic study on 105 patients aged less than one year, who underwent ductus arteriosus stenting, Qureshi et al. found a higher percentage (43%) of duct from the descending aorta, 38% from under the arch and 19% from the subclavian or innominate artery. Reference Qureshi, Goldstein and Glatz16 22% of their patients had tortuous duct (classified as ductal tortuosity type III, defined as multiple complex turns) compared to 33% in our group. In their cohort, 54% of patients had pulmonary atresia intact ventricular septum or isolated pulmonary stenosis, 42% ventricular septal defect with pulmonary stenosis/atresia, and only 5% tricuspid atresia. This correlated well with our findings that the majority of pulmonary atresia intact ventricular septum have ductus arteriosus from the descending aorta, and in biventricular lesions, ductus arteriosus are mainly from the arch of the aorta.
Patent ductus arteriosus morphology, ductal stenting and the role of CT scan
Defining ductus arteriosus morphology and pulmonary anatomy is crucial before ductal stenting to decide on the feasibility and vascular approach for stenting. Our ductal classification based on the site of origin was made as it determines the best possible approach to ductal stenting. Type II ductus arteriosus are more difficult to stent from the transfemoral arterial approach. Reference Alwi17,Reference Rehman, Marhisham and Alwi18,Reference Bauser-Heaton, Qureshi and Goldstein19 In our study cohort, about 25% of type II patent ductus arteriosus originated very proximally close to the innominate artery or type IIb (Fig 1c), and they are usually reversely orientated. This type of patent ductus arteriosus is usually best approached from the axillary or carotid artery.
Degree of ductal tortuosity is another important morphological characteristic in determining the feasibility for stenting. Generally, tortuous patent ductus arteriosus (Figs 2d-f) are referred to ductus which has multiple loops with acute turns. Reference Alwi3,Reference Qureshi, Goldstein and Glatz16 Tortuous patent ductus arteriosus are generally challenging to stent and associated with a higher failure rate. Reference Santoro, Gaio and Giugno20 However, the highly tortuous duct is now considered by many to be relative, but not absolute contraindication to ductal stenting.
The pulmonary artery anatomy is also an important factor in determining the suitability for ductal stenting. Patent ductus arteriosus stenting may cause jailing of the distal pulmonary artery branch resulting in or worsening of branch pulmonary artery stenosis, poor pulmonary artery growth, and losing the pulmonary artery if the problem is not addressed early. Reference Haranal, Mood and Leong21 Close surveillance of pulmonary artery anatomy and growth following ductal stenting is very important.
In this era, the mortality from CHDs is very low with the improvement in the accuracy of diagnosis, refinement of surgical techniques and perioperative care. Imaging modalities including echocardiogram, cardiac catheterisation, CT, and MRI serve as a live morphology study tools in congenital heart lesions. The advantages of CT over other imaging modalities include its non-invasiveness, rapid acquisition, excellent image resolution, and non-limited field view, which is safe in small, often ill neonates. Most patients only require oral chloral hydrate for sedation. In a tortuous duct, the angiogram may not be able to profile the entire ductus arteriosus clearly due to the overlapping ductal loops, which can underestimate the length and obscure the pulmonary artery anatomy. Furthermore, cardiac catheterisation is invasive and associated with significant complications including death. Reference Giordano, Santoro and Agnoletti22
Recently, rotational angiogram offers similar three-dimensional reconstruction as CT or MRI. It could serve as a roadmap to profile the ductus arteriosus, thus reducing the need for multiple angiograms and the ability to demonstrate the surrounding structures including the airway. Reference van der Stelt, Siegerink, Krings, Molenschot and Breur23 MRI provides radiation-free non-invasive morphology, as well as functional cardiac assessment. However, it involves a long scanning time, and the image resolution is inferior to the CT. Furthermore, the lung parenchyma and the airway are best imaged on CT. This study provides additional information on the risk of airway compression in a large, vertical, and tortuous duct due to its proximity to the proximal bronchus and should be routinely looked for as part of the evaluation of patent ductus arteriosus in duct-dependent pulmonary circulation. The presence of unilateral hyperinflation of the lung on the chest radiography or Chest X-Ray (CXR) warrants a CT scan to rule out airway compression by the duct. Reference Rehman, Chaudhari, Latiff, Stumper and Alwi11,Reference Markowitz, Fahey, Hellenbrand, Kopf and Rothstein24 On CT, airway compression appears as narrowing or discontinuity of the main bronchus close to any segment of the patent ductus arteriosus (Fig 4). The distal airway is usually patent as the compression in usually intermittent or dynamic in nature. In our institution, those with any degree of airway compression are sent for modified Blalock–Taussig Shunt and ductus arteriosus division.
Hence, CT is the best imaging modality to demonstrate the ductus arteriosus morphology and pulmonary artery anatomy in duct-dependent pulmonary circulation lesions. In addition, a three-dimensional printed model from CT volume dataset may aid the interventionist to strategise the procedure; however, this is quite costly and not widely available. Reference Chamberlain, Ezekian, Sturgeon, Barker, Hill and Fleming25 The success rate of ductal arteriosus stenting is about 80–93% in most centres. Reference Udink ten Cate, Sreeram, Hamza, Agha, Rosenthal and Qureshi12,Reference Santoro, Gaio and Giugno20,Reference Alwi, Choo, Latiff, Kandavello, Samion and Mulyadi26 Ductal stenting failed more often in patients with univentricular physiology and tortuous duct morphology. Reference Udink ten Cate, Sreeram, Hamza, Agha, Rosenthal and Qureshi12,Reference Santoro, Gaio and Giugno20 Therefore, in patients with tortuous ductus arteriosus assessed on echocardiogram, especially those with uni- or biventricular lesions, CT should be performed to delineate the anatomy before subjecting the patient to cardiac catheterisation. This will avoid unnecessary invasive cardiac catheterisation in those with tortuous ductus arteriosus and/or severe branch pulmonary artery stenosis, deemed not suitable for stenting. In addition, CT can be utilised to follow-up on results and complications of stenting, that is stent patency, pulmonary growth, branch pulmonary artery stenosis. Reference Alwi17,Reference Rehman, Marhisham and Alwi18
Disadvantages of CT
The main concern of CT is radiation. The radiation from CT is much lower compared to cardiac catheterisation. Gherardi et al. reported the median procedural dose range for angiography and dual-source CT was 5 (0.2–27.8) and 1.7 (0.5–9.5) millisievert, respectively (p value = 0.001). Reference Gherardi, Iball, Darby and Thomson9 With new generations of fast CT scanners and optimisation of scanning protocol, the radiation dose is minimal or submillisievert. Reference Schindler, Kehl, Wildgruber, Heindel and Schülke27,Reference Dodge-Khatami and Adebo28,Reference Schicchi, Fogante and Esposto Pirani29 Our CT protocol strictly adhered to “as low as reasonably achievable” or ALARA principles to keep the radiation to a minimum without compromising the image quality. We had no complications. Mean radiation dose using a dual-source CT for these studies was 1.3 millisievert. Thus, the benefits of obtaining valuable anatomical information in critical duct-dependent heart disease by CT overweigh its disadvantage of radiation.
Strength and weakness of this study
The strength of our study includes a large cohort study, performed on consecutive patients with various types of duct-dependent pulmonary circulation lesions. However, we do acknowledge that, we may have possibly missed a few patients during this period that underwent ductus arteriosus stenting without prior CT, for example patients with membranous pulmonary atresia intact ventricular septum planned for radiofrequency valvotomy only and those who required emergency procedure (e.g. Blalock–Taussig–Thomas shunt). Our institutional practice is biased towards ductus arteriosus stenting as the preferred palliative treatment.
What can this study add to current practice?
In patients with duct-dependent pulmonary circulation and difficulty in assessing the ductus arteriosus anatomy on echocardiogram for the suitability of ductal stenting, CT provides a valuable tool in depicting the ductus arteriosus morphology and pulmonary artery anatomy for patient selection and pre-procedural planning. This will avoid unnecessary invasive procedures in unsuitable patients. In addition, it may also be beneficial for the surgeons to decide on the site of shunt placement to the more compromised branch, lessen the risk of complications (e.g. shunt stenosis in a hypoplastic pulmonary artery or, over-shunting in a patient with large ductus) and need for concurrent pulmonary artery reconstruction.
Conclusions
CT provides a detail delineation of ductus arteriosus morphology and its effect on the pulmonary artery anatomy in duct-dependent pulmonary circulation. The key finding in this study is distinct ductus arteriosus patterns exist in the origin, degree of tortuosity, and presence of branch pulmonary artery stenosis at the site of insertion in different types of ventricle morphology. Patients with uni- and biventricular morphology have a significantly higher percentage of ductus arteriosus arising from the aortic arch (type II) and subclavian artery (type III) compared to pulmonary atresia intact ventricular septum patients, who usually have ductus arteriosus from the descending aorta (type I). However, in pulmonary atresia with intact ventricular septum with unipartite right ventricle, the majority of patients had type II patent ductus arteriosus.
This classification may be used to assess the feasibility of stenting, risk complications, and pre-procedural planning. The influence of the classification on complication, technical difficulty, or success is the suggested area that can be further studied in the future.
Acknowledgement
We would like to thank Mr Mohd Faizal Ramli, Clinical Research Department for his assistance in analysing the data.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee of Institut Jantung Negara, Kuala Lumpur, Malaysia.











