COVID-19 vaccination has been available since December 2020. On 10 May, 2021, the Food and Drug Administration approved the use of the BNT162b2 vaccine in the paediatric population aged 12 years and older. Vaccination of youth with an mRNA vaccine against SARS-CoV-2 has been recommended by the American Academy of Pediatrics and Centers for Disease Control and Prevention. 1,Reference Wallace, Woodworth and Gargano2 A single case of myocarditis in an adult following administration of the BNT162b2 vaccine has been reported from Spain. Reference García, Ortega and Fernández3 Recently, there have been several reports about myocarditis following mRNA vaccines against SARS-CoV-2. 4 We describe, for the first time, adolescents presenting with chest pain and imaging evidence of myocarditis in close temporal association with the BNT162b2 vaccination.
Two adolescent males, aged 15–16 years of age, presented in the Emergency Department with chest pain within 3 days of BNT162b2 vaccine administration, one of them after the first, the other after the second dose of the vaccine. One patient noted mild and typical vaccine-related symptoms including tactile fever, headache, and tender vaccination site within a day of vaccine administration, while the other patient had no such symptoms. Within a couple of days after vaccination, both patients developed acute onset, mid-sternal, non-radiating chest pain associated with chest tightness. One patient had a history of mild intermittent asthma, otherwise, they had no known medical conditions and no prior surgeries.
Their initial vital signs were notable for sinus tachycardia with heart rate of 108–116 beats per minute, but normal blood pressure. They had a normal cardiac exam without a murmur or friction rub. Inflammatory markers were mildly elevated (Table 1). Complete blood count was notable for neutrophilia without leukocytosis. Cardiac enzymes were elevated in both cases at presentation (Table 1). Infectious workup including immunoglobulin G and real-time reverse transcription polymerase chain reaction for SARS-CoV-2 and respiratory viral panel polymerase chain reaction containing the most common aetiologic agents of viral myocarditis were negative in both patients.
Table 1. Demographics, clinical findings, and diagnostic test results of the patients with myocarditis following BNT162b2 vaccine administration
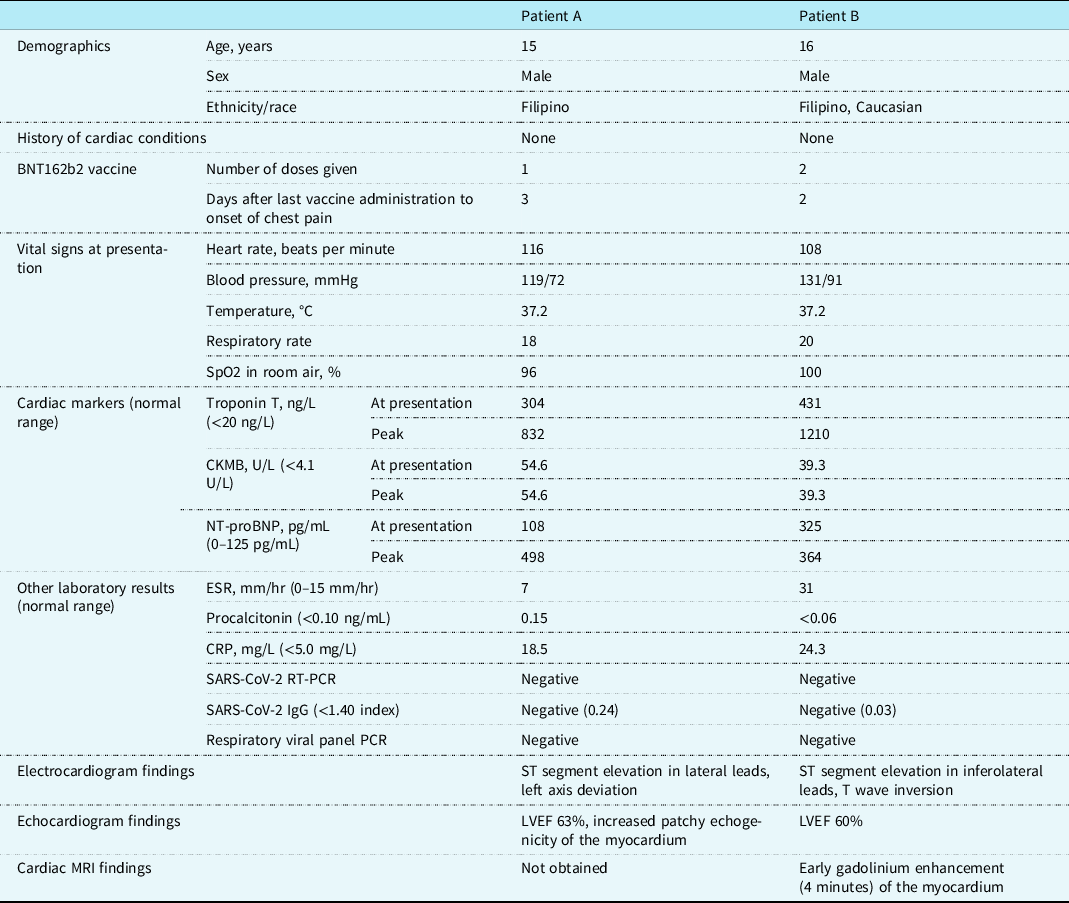
CKMB = creatine kinase myocardial band; CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; IgG = immunoglobulin G; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; PCR = respiratory viral panel polymerase chain reaction; Respiratory viral panel RT-PCR = real-time reverse transcription polymerase chain reaction; SpO2 = blood oxygen saturation.
Chest X-rays were unremarkable. Electrocardiograms showed ST elevation and T wave inversion in lateral leads (Fig 1). Both patients had normal left ventricular systolic function on echocardiogram (Table 1). Small areas of increased echogenicity could be detected throughout the myocardium in one of the patients, especially in the interventricular septum and lateral wall of the left ventricle (Fig 1). Cardiac MRI with early gadolinium enhancement using electrocardiogram-gated turbo spin echo T1-weighted sequences showed mild global early enhancement of the myocardium, with pronounced enhancement in the subendocardial layer of the left ventricle (Fig 1), findings consistent with inflammation of the myocardium without evidence of myocardial necrosis, fibrosis, or oedema. Cardiac catheterisation was not performed as there was low suspicion for acute coronary syndrome and the cardiac MRI was consistent with myocarditis.

Figure 1. Electrocardiogram, echocardiogram, and cardiac MRI findings associated with myocarditis after BNT162b2 vaccination. ( a ) Electrocardiogram shows ST segment elevation, especially in the lateral leads. ( b ) The echocardiogram shows patchy echogenic foci in the left ventricle with intact left ventricular systolic function. Arrow indicates echogenic areas in the interventricular septum. ( c ) Cardiac MRI with gadolinium enhancement using electrocardiogram-gated turbo spin echo T1-weighted sequences demonstrates early enhancement of the myocardium of the left ventricle, especially in the subendocardial layer, but no necrosis or significant oedema, consistent with myocarditis. Arrow indicates bright, enhanced areas of the left ventricular myocardium. Insert shows pre-gadolinium image with no myocardial enhancement.
One patient received intravenous immunoglobulin, while the other patient improved without any treatment. Echocardiograms continued to show normal left ventricular function throughout the hospitalisation. Troponin T peaked at 832 and 1210 ng/L, but creatine kinase myocardial band did not increase beyond initial levels (Table 1). Chest pain resolved a day after admission and both patients were discharged from the hospital within 4 days of admission. Electrocardiograms showed improved, but continued mild ST segment elevation at discharge.
Discussion
We present two adolescents with evidence of myocarditis shortly after BNT162b2 vaccination. The presentation of these adolescents were consistent with myocarditis based on clinical, imaging, and laboratory findings, and no other alternative aetiology was found. We excluded acute or recent COVID-19 infection and did not find evidence of other viral aetiologies. The temporal association with the preceding COVID-19 vaccine raised the suspicion of a vaccine-related self-limited myocarditis. Myocarditis have been rarely reported in association with vaccinations in the past. Reference Mei, Raschi and Forcesi5 BNT162b2 vaccine has certain adverse effects, including localised reaction such as swelling, pain, itchiness at the inoculation site, and generalised fatigue, headache and chills. Reference Kadali, Janagama and Peruru6 A single case report of myocarditis in a young adult after COVID-19 vaccination was reported in April 2021 from Spain, and recent reports and clinical consideration from the Centers for Disease Control and Prevention appeared during the month of May 2021. Reference García, Ortega and Fernández3,4,7 In the state of Hawaii, 33,470 adolescents aged 12–17 years have received at least one dose of BNT162b2 vaccine and of those, 10,029 adolescents have received two doses as of 1 June, 2021. 8 To our knowledge, there have been no other reported cases of myocarditis following BNT162b2 vaccine administration in the state of Hawaii in population 17 years or younger besides the two patients presented here. These reports, consistent with our findings, suggest that the BNT162b2 vaccine could rarely be associated with the development of myocarditis.
The pathomechanism of vaccine-induced myocarditis associated with the BNT162b2 vaccine is not clear. Since there is no infectious viral particle in the vaccine, the myocarditis cannot be due to an infectious agent. We hypothesise that the myocarditis is related to the immune response triggered by the vaccine in a genetically susceptible host. As the host generates antibodies against the viral particle assembled in response to the mRNA vaccine, the antibodies may cross react with surface antibodies on the cardiomyocytes resulting in an inflammatory reaction with cell damage. This cell damage, manifesting as focal patchy myocarditis, causes the ST segment elevation and chest pain. Based on our limited experience and recent vaccine-related news reports, it is possible that myocarditis in temporal association with the BNT162b2 vaccine is an important side effect to monitor. Myocarditis associated with the current mRNA vaccine seems self-limited without any circulatory collapse. Our case series suggests that chest pain within a week of COVID-19 vaccination with an mRNA vaccine should raise the suspicion of focal myocarditis. Although this focal myocarditis may be self-limited and resolve without intervention, ST segment elevation and troponin elevation may occur, and patients should be monitored for the extent of cardiac involvement, symptoms, and cardiac function. These patients with vaccine-related myocarditis should refrain from physical activity following general guidelines for myocarditis. Reference Maroon, Udelson and Bonow9
Acknowledgements
We would like to acknowledge Dr. Marian Melish and Dr. Sanah Christopher for their contribution in the management of patients included in this report.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Appropriate consent was obtained from the patients’ families. The current report is exempt from Institutional Review Board review.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951121002547





