Chronic kidney disease (CKD) is a major complication of type 2 Diabetes Mellitus (T2DM). Approximately a third of T2DM patients will develop CKD, increasing their risk of all-cause mortality by 3–19 %( Reference Pugliese, Solini and Bonora 1 ). Polyphenolics are dietary factors potentially able to prevent damage in the kidney, through inhibition of glycation, reduction of uremic damage, and increased radical-scavenging activity( Reference Khangholi, Fadzilah and Berwary 2 ). Polyphenolics-rich products (including fruit and vegetables) are sometime restricted in CKD patients to reduce the risk of hyperkalaemia (serum >5·5 mM), but it is not clear how widespread this is. In the early stages of CKD (GFR > 30 mL/min/1·72 m2), a diet high in fruits and vegetables may delay CKD progression( Reference Snelson, Clarke and Coughlan 3 ); however, studies about the dietary habits of CKD patients (before dialysis) are lacking and hamper recommendations in this patient group. The aim of the present study was to estimate polyphenolics and potassium intake in T2DM patients with CKD stage 3, and in matched healthy controls.
The cross-sectional study included 87 T2DM subjects with CKD stage 3 as cases and 87 healthy adults matched by age and gender as controls. Polyphenolics and potassium intake was estimated using the EPIC-Norfolk food frequency questionnaire and liquids intake with a beverage questionnaire. The Kawasaki formula( Reference Kawasaki, Ithoh and Uezono 4 ) was used to estimate 24-hour urinary potassium excretion from a spot urine sample.
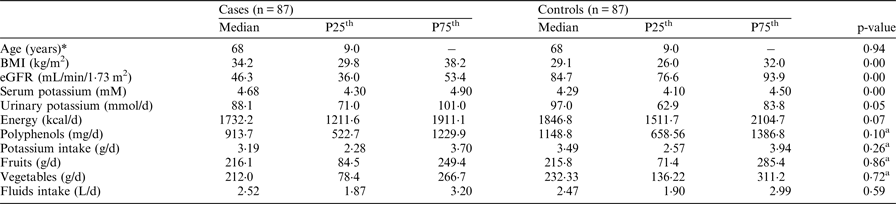
*Data is normally distributed and presented as mean and standard deviation. p-values between-group comparisons were calculated using Mann-Whitney U Test. aAdjusted for energy intake.
Potassium intake should only be limited if blood tests shows it to be necessary; it was the case in only 8 % (n = 7) T2DM patients (5·5–6·1 mM); with a further 17 % (n = 15) under angiotensin-converting enzyme inhibitors that may precipitate hyperkalaemia. There are no specific dietary recommendations for patients with early CKD; 18 % (n = 16) reported to restrict dietary potassium and 30 % (n = 26) had visited a dietitian at least once in the preceding year. Energy intake was below the adult reference intake (2,000 kcal/d) and is likely to indicate underreporting - 70 % (n = 61) of CKD subjects did not meet general dietary recommendation for potassium (RNI 3·5 g/d) and 57 % (n = 50) for fruit and vegetable intake (400 g/d)( Reference Thomas 5 ), compared to 40 % in controls who did not meet the recommendations for both. Fruits and vegetable intake contributed toward 28 % of the total potassium intake followed by dairy products (18 %) and meat products (12 %). T2DM patients' potassium excretion was 9 % lower than controls. Some fruits and vegetables are low in potassium, high in polyphenols and could represent an opportunity for intervention. Long-term trials in early CKD patients investigating polyphenol and potassium intake on renal biomarkers and clinical outcomes are needed to address dietary recommendations and reduce the progression of the disease.
S.A.P-D acknowledges PhD scholarship funding by Mexican National Council for Science and Technology.


