This abstract was awarded the student prize for best oral original communication.
Vitamin D deficiency is highly prevalent in the UK and Ireland and is defined as a total 25-hydroxyvitamin D (25[OH]D) concentration below 30 nmol/L with respect to bone health(Reference Ross, Manson and Abrams1). Owing to the UK and Ireland's northerly latitudes (50–58 and 51–55°N respectively) as well as the limited range of naturally occurring and fortified dietary sources of vitamin D, supplementation is often regarded as advisable in order to optimise wintertime vitamin D status. Interventions typically use capsules as a peroral method of delivery. This study aimed to compare the efficacy of two forms of supplemental vitamin D3; liquid capsules or oral spray solution, at increasing total 25(OH)D concentrations in healthy adults.
In total, 22 participants (males n = 10 and females n = 12) were independently randomised to receive 3000IU (75μg) vitamin D3 daily for 4 weeks in either a capsule or oral spray form during wintertime (Oct–Feb). Following a 10-week washout, participants crossed-over onto the opposite treatment for a final 4 weeks. Height (cm) was measured at baseline while weight (kg) and fasted blood samples were obtained before and after each supplementation phase. Total 25(OH)D was quantified using LCMS-MS and intact parathyroid hormone (PTH) concentration was measured by ELISA. Dietary vitamin D intake was estimated using a validated food frequency questionnaire(Reference Weir, Carson and Mulhern2).
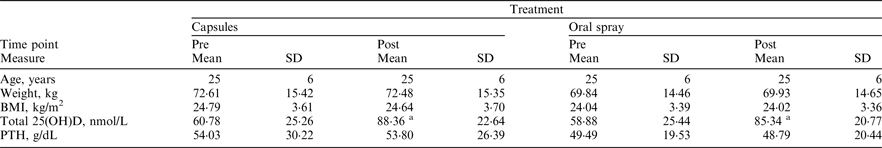
asignificantly different from pre-intervention P < 0·05 (paired t test)
Overall, baseline mean ± SD total 25(OH)D concentration averaged 59·76 ± 29·88 nmol/L, representing clinical sufficiency. Prior to hypothesis testing, a time by treatment interaction and potential carryover effects were ruled-out (P = 0·107 and P = 0·681, respectively). Subsequently, analysis of covariance determined that there was no significant difference in mean ± SD change from baseline, with respect to total 25(OH)D concentrations, between oral spray and capsule supplementation (26·46 ± 23·91 versus 27·58 ± 15·93 nmol/L respectively, P = 0·995). Dietary vitamin D intake averaged 6·25 ± 6·24μg/day, falling short of the current 10μg/day reference nutrient intake. Our findings advocate oral spray vitamin D3 supplementation as an equally effective alternative to capsules. This may have major implications for micronutrient delivery in those with malabsorption syndromes; as vitamin D3 administered by oral spray bypasses the intestine via buccal, sublingual and palatal membrane absorption sites in the oral cavity. This supplementation method will also prove advantageous for those with difficulty swallowing such as the elderly, young children and babies.
This study was funded by the Department for Employment and Learning, Northern Ireland and Translational Research Group: Diabetes, Endocrinology & Nutrition, HSC Research & Development Division, Public Health Agency, Belfast. Oral spray solutions were gifted by BetterYou Ltd. Ethical approval was obtained from the University of Ulster Research Ethics Committee (REC/15/0083) and the study was conducted according to the guidelines laid down in the Declaration of Helsinki.


