Individuals with schizophrenia or bipolar disorder have life expectancies that are 15–20 years shorter than average.Reference Laursen, Wahlbeck, Haellgren, Westman, Oesby and Alinaghizadeh1 Autopsies indicate that the most common cause of sudden death in patients with schizophrenia is cardiovascular disease, especially myocardial infarction.Reference Ifteni, Correll, Burtea, Kane and Manu2, Reference Sweeting, Duflou and Semsarian3 Compared with age- and gender-matched controls, patients with schizophrenia or bipolar disorder are at least twice as likely to develop type 2 diabetes,Reference Stubbs, Vancampfort, De Hert and Mitchell4, Reference Vancompfort, Mitchell, De Hert, Sienaert, Probst and Buys5 which is a risk factor for cardiovascular disease.Reference Sarwar, Gao, Seshasai, Gobin, Kaptoge and Di Angelantonio6 Some antipsychotic medications including second-generation antipsychotics can lead to substantial weight gain,Reference Leucht, Corves, Arbter, Engel, Li and Davis7 which increases the risk of dyslipidaemia and diabetes.Reference Graham, Cho, Brownley and Harp8, Reference Newcomer9 Thus, patients with schizophrenia or bipolar disorder who are receiving antipsychotics should be appropriately monitored for the development of cardiovascular risk factors such as obesity and diabetes.
Few cross-sectional studies have examined the prevalence of glucose abnormalities in patients with schizophrenia.Reference Kusumi, Ito, Honda, Hayashishita, Uemura and Hashimoto10–Reference Van Winkel, De Hert, Van Eyck, Hanssens, Wampers and Scheen12 Cross-sectional studies are relatively easy to perform and permit the recruitment of many participants, but they do not clearly establish causality. Ideally, longitudinal pharmacogenetic studies of metabolic effects should recruit hundreds or thousands of patients and follow them for years, but doing so is difficult and expensive.Reference de Leon and Diaz13 Prospective data from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial indicated that of the second-generation antipsychotics, olanzapine causes the most metabolic side-effects and ziprasidone causes the least.Reference Meyer, Davis, Goff, McEvoy, Nasrallah and Davis14 These results confirmed that second-generation antipsychotics differ in their metabolic impacts. We previously conducted a longitudinal study of glucose abnormalities in patients with schizophrenia treated with second-generation antipsychotics,Reference Kusumi, Ito, Uemura, Honda, Hayashishita and Miyamoto15 but that study had several limitations, including a retrospective design, the inclusion of patients who were not starting a new antipsychotic at the beginning of the study, a lack of medication history or monitoring of co-administered drugs during the pre-entry and study periods, the exclusion of patients receiving first-generation antipsychotics and recruitment from a small geographic area. We thus sought to conduct a more sophisticated study to overcome these limitations. Several countries have recently developed guidelines for the routine monitoring of body weight, serum lipids and blood glucose in patients with schizophrenia.Reference Cohn and Sernyak16, 17 These guidelines are expected to improve the detection and prevention of diabetes and other glucose abnormalities. We similarly proposed a method for monitoring blood glucose in patients with schizophrenia receiving second-generation antipsychotics in Japan.Reference Kusumi, Ito, Honda, Hayashishita, Uemura and Hashimoto10 However, few guidelines have been proposed to prevent glucose-related abnormalities in patients with bipolar disorder. Accordingly, we conducted a nationwide, multisite, 1-year prospective cohort study using the Japanese blood glucose monitoring guidelines in order to identify predictive factors for hyperglycaemia in patients treated with antipsychotics who have schizophrenia, schizoaffective disorder or bipolar disorder. We also examined the effects of antipsychotics on hyperglycaemic progression to test our hypothesis that regular monitoring is necessary even in patients taking low-risk antipsychotics.
Method
Study population
Individuals were diagnosed with schizophrenia, schizoaffective disorder or bipolar disorder based on the criteria in ICD-10.18 The inclusion criteria were initiation of a first- or second-generation antipsychotic medication (either by changing medications or adding a new medication), a 1-year medication history prior to enrolment, no diagnosis of diabetes prior to baseline screening and not being classified as probable diabetes at baseline monitoring. Participant selection was consecutive at each site. All participants provided written informed consent after receiving a full explanation of the study procedures.
Study design
Participants were enrolled between April 2013 and March 2015 and followed-up for 1 year based on the Japanese blood glucose monitoring guidelines for patients with schizophrenia.Reference Kusumi, Ito, Honda, Hayashishita, Uemura and Hashimoto10 The study was conducted at 44 sites (24 general hospitals, 17 psychiatric hospitals and 3 psychiatric clinics) throughout Japan, was approved by each site's institutional review board and conformed to the principles of the Declaration of Helsinki. Data were collected through an electronic database system (CapTool Prime; Mebix, Tokyo, Japan) and managed at the Hokkaido University Hospital Clinical Research and Medical Innovation Center. For thorough follow-up data collection, researchers received notices about missing data from the data management centre when the monitoring period was over.
To identify predictive factors for hyperglycaemic progression in patients with normal or prediabetic baseline glucose levels, we first examined the number of patients who progressed from normal glucose levels to prediabetes or probable diabetes and the number who progressed from prediabetes to probable diabetes during the 1-year follow-up period. We then conducted a Cox regression analysis using demographic data and monitoring measurements. Moreover, to examine the effects of antipsychotics on hyperglycaemic progression during the follow-up period, we compared how frequently classifications became at least one step worse (i.e. from normal glucose levels to prediabetes or probable diabetes, or from prediabetes to probable diabetes) among patients receiving monotherapy with any of the six antipsychotics most frequently used in our study.
Assessments
In the initial screenings, we obtained participant demographic characteristics including age, gender, illness duration, out-patient versus in-patient status, smoking status, drinking status, familial disease histories (including schizophrenia, bipolar disorder, major depressive disorder, diabetes mellitus and dyslipidaemia), coexisting medical diagnoses (including hypertension, heart disease and dyslipidaemia), and 1-year medication histories prior to enrolment and during the study period. Before the initiation of a new antipsychotic, we obtained baseline measurements of blood glucose (fasting or postprandial) or glycated haemoglobin (HbA1c), serum lipids (total cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides), weight, body mass index (BMI) and clinical diabetic symptoms such as dry mouth, excessive fluids consumption, cravings for sugary drinks, polyuria and frequent urination.
According to the Japanese guidelines for blood glucose monitoring in patients with schizophrenia,Reference Kusumi, Ito, Honda, Hayashishita, Uemura and Hashimoto10 patients' blood glucose measurements were classified as normal, prediabetic or probably diabetic. Normal was defined as fasting blood glucose <110 mg/dL, postprandial blood glucose <140 mg/dL or HbA1c <6.0%; prediabetes was defined as fasting blood glucose of 110–125 mg/dL, postprandial blood glucose of 140–179 mg/dL or HbA1c of 6.0–6.4%; and probable diabetes was defined as fasting blood glucose >125 mg/dL, postprandial blood glucose >179 mg/dL or HbA1c >6.4%. Because these classifications permit the early detection of possible diabetes, declassification is never allowed even if normal measurement values are recovered. The follow-up measurements were also scheduled according to the Japanese monitoring guidelinesReference Kusumi, Ito, Honda, Hayashishita, Uemura and Hashimoto10 and were conducted at months 3, 6 and 12 in patients with normal glucose levels; months 1, 3, 6, 9 and 12 in patients with prediabetes; and every month in patients with probable diabetes.
Statistical analysis
We used a Cox proportional-hazards regression modelReference Cox19 to identify predictive factors for hyperglycaemic progression. It accounted for demographic variables including gender; age; diagnosis (schizophrenia/schizoaffective disorder versus bipolar disorder); duration of illness; treatment status (out-patient versus in-patient); smoker status; drinker status; familial histories of schizophrenia, bipolar disorder, major depression, diabetes and heart disease; coexisting diagnoses of dyslipidaemia, hypertension and heart disease; baseline measurements including weight, BMI (< 25 v. ≥25), total cholesterol (< 220 v. ≥220 mg/dL), HDL-cholesterol (< 40 v. ≥40 mg/dL) and triglycerides (< 150 v. ≥150 mg/dL); clinical diabetes symptoms such as dry mouth, excessive fluids consumption, craving for sugary drinks, polyuria and frequent urination; and medications at baseline (second- or first-generation antipsychotics pre-administrated with a newly initiated antipsychotic drug). Statistical significance was evaluated with likelihood ratio and hazard ratio (HR) tests with 95% profile likelihood confidence interval.
To examine the effects of antipsychotic monotherapy on hyperglycaemic progression, we estimated the hyperglycaemic progression rate as 15% based on our previous study.Reference Kusumi, Ito, Uemura, Honda, Hayashishita and Miyamoto15 For a two-sided confidence interval of a binomial proportion whose true value was 0.15, a sample size of 196 yielded a maximal half-width of 0.05. We estimated that 40% of patients used one of the six most commonly used second-generation antipsychotics and that 50% of patients continued monotherapy for more than 10 months. Since 10% of participants were on a first-generation antipsychotic, the minimum necessary sample size was estimated at 1089.
To examine the effects of antipsychotics on hyperglycaemic progression, we selected patients with normal or prediabetic baseline glucose levels who received antipsychotic monotherapy for more than 10 months. We used the two -sided Fisher's exact test to determine whether the baseline frequencies of prediabetes and hyperglycaemic progression rates during the 1-year period depended on the antipsychotic used among patients receiving monotherapy with any of the six most frequently used antipsychotics in this study. Statistical significance was defined as P < 0.05. Analyses were conducted using JMP Pro 13.1.0 (SAS Institute, Cary, NC).
Results
Participants
We performed inclusion screenings on 1323 patients with schizophrenia, schizoaffective disorder or bipolar disorder who had started treatment with a first- or second-generation antipsychotic. Of them, 77 declined to participate, 41 failed to meet the inclusion criteria, and 3 were rejected as duplicate enrolments. Because 36 patients who were classified as probably diabetic at baseline monitoring were removed from analysis, the final sample included 1166 patients (512 men and 654 women; mean age 48.4 years, s.d. = 16.7) whose various characteristics are shown in Table 1. Of the participants, 982 (84.2%) were diagnosed with schizophrenia or schizoaffective disorder and 184 (15.8%) were diagnosed with bipolar disorder. Of the six antipsychotics examined, aripiprazole was the most frequently prescribed as a starting drug (25.6%), followed by olanzapine (16.6%). At the study's initiation, 540 patients (46.3%) started treatment with antipsychotic monotherapy.
Table 1 Participant characteristics and baseline monitoring and medication

HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin–noradrenalin reuptake inhibitor.
a. There is missing detail for some variables as indicated.
Blood glucose classifications
At baseline, 1042 patients (89.4%) were normal and 124 (10.6%) were prediabetic (Table 1). In total, 1018 participants (87.3%) completed the 1-year follow-up period, and their glucose level classification changes are shown in Table 2. Of the 1042 patients whose results were initially normal, 116 became prediabetic (12.6%) and 20 became probably diabetic (2.2%). Of the 124 patients who were initially prediabetic, 18 became probably diabetic (18.8%).
Table 2 Changes in prediabetes and probable diabetes rates during the 1-year follow-up period
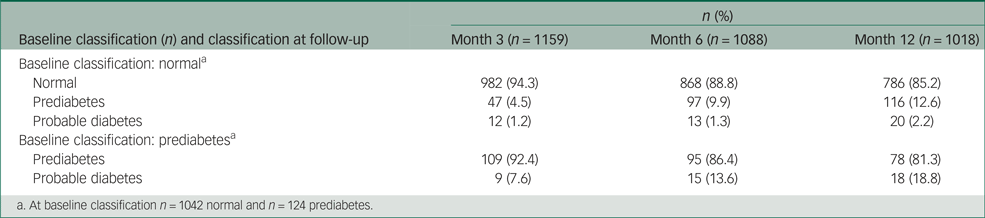
a. At baseline classification n = 1042 normal and n = 124 prediabetes.
Predictive factors for hyperglycaemic progression
The simple Cox regression analysis identified significant predictive factors including age (HR = 1.02, 95% CI 1.01–1.02, P = 0.001); familial histories of schizophrenia (HR = 0.65, 95% CI 0.38–1.04, P = 0.007); coexisting dyslipidaemia (HR = 1.69, 95% CI 1.15–2.42, P = 0.008), hypertension (HR = 1.93, 95% CI 1.30–2.78, P = 0.002) and heart disease (HR = 2.09, 95% CI 1.15–3.47, P = 0.017); and baseline BMI (HR = 1.39, 95% CI 1.02–1.87, P = 0.037) and serum triglycerides (HR = 1.62, 95% CI 1.16–2.23, P = 0.005) (Table 3). The multivariate Cox regression analysis indicated that coexisting hypertension (HR = 1.80, 95% CI 1.01–3.13, P = 0.048) and baseline serum triglycerides (HR = 1.94, 95% CI 1.22–3.03, P = 0.006) were significant predictors of hyperglycaemic progression during the study period (Table 3).
Table 3 Cox regression analysis for predictive factors of hyperglycaemic progression in patients with normal or prediabetic baseline glucose levels

HDL, high-density lipoprotein.
Effects of antipsychotics on hyperglycaemic progression
Among the patients who were taking any of the six most frequently used antipsychotics, there were no significant between-antipsychotic differences in the frequencies of baseline prediabetes (aripiprazole, 10%; olanzapine, 11%; quetiapine, 9%; risperidone, 23%; perospirone, 13%; blonanserin, 11%; P = 0.67) or the hyperglycaemic progression rates over the study period (aripiprazole, 15%; olanzapine, 20%; quetiapine, 26%; risperidone, 5%; perospirone, 13%; blonanserin, 22%; P = 0.42) (Table 4).
Table 4 Hyperglycaemic progression in patients treated with antipsychotic monotherapy

a. Fisher's exact test (2-sided): P = 0.42.
Discussion
Principal findings
We aimed to identify clinical predictors for hyperglycaemic progression in patients treated with antipsychotics who had schizophrenia, schizoaffective disorder or bipolar disorder, and we identified elevated serum triglycerides and coexisting hypertension as such predictors. By comparing hyperglycaemic progression rates among patients receiving the six most frequently used antipsychotics in this study, we also confirmed our hypothesis that comprehensive longitudinal monitoring is essential in regular clinical practice irrespective of the antipsychotic used.
Hypertension and diabetes
Some cross-sectional studies have suggested a relationship between hypertension and diabetes in the general population,Reference Henry, Thomas, Benetos and Guize20, Reference Iimura21 but prospective cohort studies have reported conflicting findings about whether individuals with hypertension are at an elevated risk for developing type 2 diabetes.Reference Janghorbani and Amini22–Reference Gress, Nieto, Shahar, Wofford and Brancati24 In non-diabetic first-degree relatives of patients with type 2 diabetes, individuals with hypertension were no more likely to progress to type 2 diabetes than individuals without hypertension were.Reference Janghorbani and Amini22 A prospective large-cohort Turkish study indicated that type 2 diabetes was significantly predicted by prehypertension (i.e. systolic blood pressure of 120–139 mmHg or diastolic blood pressure of 80–89 mmHg) in women (relative risk 2.06) but not in men.Reference Onat, Yazici, Can, Kaya, Bulur and Hergenc23 A prospective cohort study of representative individuals aged 45–64 years suggested that type 2 diabetes was almost 2.5-fold more likely to develop in individuals with hypertension than in individuals with normal blood pressure.Reference Gress, Nieto, Shahar, Wofford and Brancati24 Hypertension and diabetes share many aetiological pathways with conditions such as obesity, inflammation, oxidative stress and insulin resistance.Reference Cheung and Li25 This study is the first to indicate that coexisting hypertension predicts diabetic progression in patients treated with antipsychotics who have schizophrenia or bipolar disorder.
Diabetic progression during the follow-up period
Of the patients with normal baseline glucose levels, 12.6 and 2.2% were reclassified as having prediabetes and probable diabetes, respectively, over the 1-year follow-up period (Table 2). These rates are consistent with those of our previous study,Reference Kusumi, Ito, Uemura, Honda, Hayashishita and Miyamoto15 but the rate of progression from prediabetes to probable diabetes was much lower in the present study (18.8%) than in our previous study (42.4%). This may be because a greater proportion of participants completed the 1-year follow-up period in this study (1018 out of 1166, 87.3%) than in our previous study (374 out of 537, 69.6%). Our previous study's results might have been more subject to bias because of missing data. The current study had fewer missing data, probably because of the systematic feedback system for physicians that included reminders from the data management centre to report complete 1-year follow-up data. Because the physicians were thus prompted to monitor their patients more thoroughly, they were probably more likely to discover prediabetic states and encourage healthy diets and exercise as necessary. This could have prevented progression from prediabetes to probable diabetes. These results suggest that strict longitudinal monitoring is important for predicting and identifying the progression of diabetes and other glucose abnormalities in patients treated with antipsychotics who have schizophrenia or bipolar disorder.
Effect of antipsychotics on diabetic progression
In this study, hyperglycaemic progression rates over the 1-year observation period did not significantly differ among the six most frequently used antipsychotics. This finding can be explained by noting that this is an observational study, not a randomised controlled study, and that clinicians usually prescribe low-risk drugs to patients at high risk for diabetic progression. In contrast to the results of the CATIE study,Reference Meyer, Davis, Goff, McEvoy, Nasrallah and Davis14 these prescription biases might have reduced our ability to identify diabetic progression induced by high-risk antipsychotics such as clozapine and olanzapine and increased the apparent risk associated with low-risk antipsychotics such as aripiprazole.17 Thus, irrespective of the antipsychotic used, comprehensive longitudinal monitoring is essential in regular clinical practice.
Strengths and limitations of the study
Important strengths of this study were its nationwide, relatively large sample; strict longitudinal monitoring based on the Japanese guidelines in real clinical settings; and its examination of the effect of antipsychotics on diabetic progression. Although various limitations of our previous studyReference Kusumi, Ito, Uemura, Honda, Hayashishita and Miyamoto15 were overcome in the present study, a 1-year follow-up period might have been insufficient for observing diabetic progression. Furthermore, our analyses of the effects of specific antipsychotics on hyperglycaemic progression relied on data from only a subset of the patient sample because most patients took more than one antipsychotic for at least a short period during this study. Relatively few Japanese people are severely obese,26 but even mild obesity may lead to hyperglycaemia in Japanese individuals.Reference Yoshiike, Nishi, Matsushima, Ito, Ikeda and Kashihara27 Therefore, our results may not be generalisable to Western populations, but our study's focus on a non-Western population is also a strength because few studies have been conducted outside the USA and Europe. Future studies should use longer follow-up periods and larger samples.
Implications for clinical practice and research
High baseline serum triglycerides and coexisting hypertension are important predictors of diabetic progression in patients treated with antipsychotics who have schizophrenia or bipolar disorder. Irrespective of the antipsychotic used, comprehensive longitudinal monitoring is essential in regular clinical practice.
Funding
This study was supported by Early-Phase/Exploratory or International-Standard Clinical Research grants from the Japan Agency for Medical Research and Development (16lk0103005h0005). The funding sources had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Acknowledgements
We thank the following people for substantial contributions to data acquisition: Drs H. Narita, Y. Nakato, S. Nakagawa, Y. Shimizu, T. Inoue, T. Saito, K. Kitagawa, Y. Fujii, S. Asakura, K. Toyoshima, R. Kameyama, Y. Wakatsuki, Y. Mizukami, Y. Hayashishita, and T. Tanaka of the Hokkaido University Graduate School of Medicine Department of Psychiatry (Sapporo, Japan); Drs S. Ikezawa, N. Kuroki, M. Ohmori, and K. Nakagome of the National Center of Neurology and Psychiatry Department of Psychiatry (Kodaira, Japan); Drs H. Muraoka, T. Kohno, K. Takahashi, and J. Ishigooka of the Tokyo Women's Medical University Department of Psychiatry (Tokyo, Japan); Drs H. Watanabe and M. Iyo of the Chiba University Department of Psychiatry (Chiba, Japan); Dr S. Miyamoto of the St. Marianna University School of Medicine Department of Psychiatry (Kawasaki, Japan); Dr K. Hatta of the Juntendo University Nerima Hospital Department of Psychiatry (Tokyo, Japan); Drs S. Ozaki and M. Okumura of the Toshima Hospital Department of Psychiatry (Tokyo, Japan); Drs T. Kikuchi and K. Watanabe of the Kyorin University Department of Psychiatry (Mitaka, Japan); Drs H. Yoshino, S. Ueda, K. Ikeshita, Y. Nakanishi, and T. Kishimoto of the Nara Medical University Department of Psychiatry (Nara, Japan); Drs Y. Koshikawa and T. Kinoshita of the Kansai Medical University Department of Neuropsychiatry (Moriguchi, Japan); Drs S. Watabe, H. Kubo, N. Kameoka, T. Tominaga, and T. Ohmori of the Tokushima University Department of Psychiatry (Tokushima, Japan); Drs K. Kawabe, N. Sonobe, Y. Miyama, H. Shimizu and S. Ueno of the Ehime University Department of Psychiatry (Matsuyama, Japan); Drs A. Kozuki, R. Yoshimura, A. Sugita and H. Hori of the University of Occupational and Environmental Health Department of Psychiatry (Kitakyusyu, Japan); Dr T. Ueno of the Hizen Psychiatric Center (Kanzaki, Japan); Drs Y. Yada, K. Bessho, T. Horikoshi, Y. Mitsui, H. Itakura, Y. Kokoroishi, K. Sato, M. Fujiwara, M. Chida, R. Sou, M. Takase, K. Makino, Y. Kishi, T. Sunami, S. Ikeda, T. Kohno, and T. Miyake of the Okayama Psychiatric Medical Center (Okayama, Japan); Dr K. Suzuki of the Aomori Prefectural Central Hospital Department of Psychiatry (Aomori, Japan); Drs H. Tanii and M. Okada of the Mie University Department of Psychiatry (Tsu, Japan); Drs T. Hirooka and H. Miyaoka of the Kitazato University East Hospital Department of Psychiatry (Sagamihara, Japan); Drs K. Fujita, I. Yasue, S. Tanaka, M. Hattori, A. Okuda, R. Suzuki, K. Miyahara, M. Yonehara, R. Tsuji, G. Cho, K. Okada, N. Kimura, M. Takagi, T. Mekata, M. Hirano, and S. Takeichi of the Okehazama Hospital (Toyoake, Japan); Drs R. Matsubara, Y. Usukubo, R. Nozawa, and T. Fujie of the Sapporo Hanazono Hospital (Sapporo, Japan); Drs S. Iizuka, M. Sato, T. Isoyama, C. Ikeda, A. Mikami, and Y. Hyakkan of the Hakodate Watanabe Hospital (Hakodate, Japan); Drs N. Okamoto, Y. Niwa, and T. Suzuki of the Sapporo Suzuki Hospital (Sapporo, Japan); Dr K. Nakajima of the Yakumo General Hospital Department of Psychiatry (Yakumo, Japan); Dr S. Tsuchida of the Kutchan-Kosei General Hospital Department of Psychiatry (Kutchan, Japan); Drs Y. Kamikawa and N. Morita of the Kei-ai Hospital (Noboribetsu, Japan); Dr T. Takeuchi of the Tomakomai Midorigaoka Hospital (Tomakomai, Japan); Drs H. Takeshige and T. Maeda of the Hokkaido Koyogaoka Hospital (Abashiri, Japan); Drs T. Egashira, M. Oka, and H. Umezu of the Wakkanai City Hospital Department of Psychiatry (Wakkanai, Japan); Drs A. Ito and A. Mikami of the Muroran City General Hospital Department of Psychiatry (Muroran, Japan); Drs S. Miyano and H. Sawayama of the Teine Hospital (Sapporo, Japan); Drs T. Ishikane, M. Kohsaka, and H. Mieda of the Ishikane Hospital (Sapporo, Japan); Dr T. Hayashishita of the Hayashishita Hospital (Sapporo, Japan); Dr T. Komiyama of the Iida Hospital (Iida, Japan); Drs Y. Oyanagi, H. Honma, M. Okumura, Y. Hosokawa, Y. Umemoto, and T. Horinouchi of the Obihiro National Hospital Department of Neuropsychiatry (Obihiro, Japan); Dr I. Suzuki of the Sapporo Kokoronomori Clinic (Sapporo, Japan); Dr T. Matsuyama of the Okamoto Hospital (Sapporo, Japan); Dr Y. Maki of the Maki Hospital (Iwamizawa, Japan); and Drs T. Tao and J. Watanabe of the Oyachi Hospital (Sapporo, Japan). The following project staff from the Hokkaido University Hospital Clinical Research and Medical Innovation Center provided outstanding support for this project: T. Miyakoshi, A. Hirai, S. Tanno, C. Nishimura, and C. Asano, who assisted with database management, and T. Amano and K. Ono, who assisted with statistical analysis. Editage (www.editage.jp) assisted with English-language editing.







eLetters
No eLetters have been published for this article.