Patent ductus arteriosus closure with the transcatheter method is a treatment modality that has been used for many years. However, transcatheter closure of a duct requires a device suitable for the specific ductal anatomy and compatible with the age and weight of the patient. Reference Baruteau, Hascoet and Baruteau1–Reference Delaney and Fletcher3 Therefore, the development of new devices and modifications of the available ones were required, particularly for children weighing less than 4 kg and premature newborns. In small children, problematic structural properties of ductus occluders include the size of the delivery systems, stifness of the delivery cables, and dimension of the aortic and/or pulmonary discs. Reference Kenny, Morgan and Bentham4–Reference Baspinar, Sahin and Sulu6 The use of Amplatzer Piccolo Occluder (formerly known as Amplatzer ductal occluder II additional sizes), a third-generation ductal occluder developed by Amplatzer (St. Jude Medical (Abbott), Plymouth, Minnesota, USA) has become more and more common. Reference Narin, Pamukcu, Baykan, Sunkak, Ulgey and Uzum7,Reference Sungur, Karakurt, Ozbarlas and Baspinar8 Due to its symmetric structure, properties of the discs, ability to close ductus of up to 4 mm diameter, and a delivery sheath size of only 4F, the Piccolo device was approved by the FDA in January 2019 for use in duct closure in patients weighing ≥ 700 g. Reference Sathanandam, Gutfinger and O'Brien9
This study aimed to provide a retrospective evaluation of patent ductus closure’s medium to long-term safety and efficacy with the Piccolo device in 645 patients consisting of premature infants, babies, and children who underwent transcatheter ductus closure at five paediatric cardiology centres in Turkey.
Method
A total of 645 children underwent patent ductus arteriosus closure with the Piccolo via the transcatheter approach at five Pediatric Cardiology Centers between 2016 and 2021. Patients with comorbid cardiac abnormalities that would require emergency surgical intervention were excluded. Informed consent was obtained from the family of each patient. Ethics committee approval was obtained with code 2021/11-24.
Retrospective data analysis was performed for procedural success, clinical characteristics, complications, and demographic data. Since the study also included premature infants with very low birth weight, the data were divided into groups ≤ 1.5 kg, 1.5–3 kg, and > 3 kg.
Device description
Amplatzer Piccolo Occluder is a self-expanding device made from a nitinol wire mesh and consists of a central waist and retention discs placed on either end. The discs of the device are 1 mm, 1.25 mm, and 1.5 mm larger than the waist (Supplemantary Figure S1). The device can be delivered through the 4F delivery catheter via the aortic or venous route. The central waist is designed to fill the ductal lumen, and the retention discs are designed to be placed on the pulmonary and aortic ends of the ductus arteriosus.
Technique
Although the procedure was performed under general anaesthesia in newborns, older patients mostly required sedation and local anaesthesia with haemodynamic monitoring. The only access route was the femoral vein in premature babies and those < 2 kg. The ductal structure was viewed in the lateral and right oblique positions. The device size was 1–2 mm larger than the central diameter in preterm infants and generally 1–3 mm larger than the pulmonary end in non-preterm infants, particularly those with a conic duct. Occasionally, devices with a disc that could fill the ampulla were selected for small ductus. Ductal anatomy was assessed according to the classification by Krichenko et al, Reference Krichenko, Benson, Burrows, Moes, McLaughlin and Freedom10 except for the foetal type.
In preterm babies, shunt measurement was not performed during catheterisation due to the risk of blood loss. The aortic end was identified using markers such as an oesophageal feeding tube, heat probe, and tracheal air column in premature babies. Preterm ductus was accessed through the right ventricle and pulmonary artery with a 4F Judkins right catheter or a Cobra 2 glide catheter with the aid of a 0.014-inch floppy coronary wire in front of tricuspid valve. Frequently 4F Judkins right catheter, coronary wire, and Y-connector were used together to avoid any tricuspid chordal damage with gentle movement of the wire from the tricupid valve through to patent ductus arteriosus. Sometimes to prevent any iatrogenic tricuspid regurgitation, a microcatheter were needed. The contrast medium was manually injected using the side arm placed on the delivery system catheter to verify the device location before release. Intraductal device placement was preferred in premature babies, in addition to the use of shorter devices. Moreover, left pulmonary artery or descending aortic obstruction was evaluated by transthoracic echocardiography in the venous approach group. Highly echocardiographic visibility of the device provided a clear advantage to evaluate efficacy of the procedure. An echocardiogram was performed for review purposes on postoperative day 1, and other follow-up tests were performed according to the protocols employed at the centres.
Statistical analysis
The data were analysed with the SPSS Statistics Standard Concurrent User V 26 (IBM Corp., Armonk, New York, USA) statistical software package. Descriptive statistics were expressed with the number of units (n), percentage (%), mean ± standard deviation (
![]() ${\bar{x}}$
± sd), median (M), minimum (min), and maximum (max) values. The normal distribution of numerical variables was evaluated with the Shapiro–Wilk test for normality. Numerical variables were compared according to weight groups with the Kruskal–Wallis test. In the Kruskal–Wallis analysis, the Dunn-Bonferroni test was used for multiple comparisons. Categorical variables were compared according to groups with Fisher’s exact test. When Fisher’s exact test yielded significant results, intergroup differences were evaluated using the two-sample z test with Bonferroni correction for each category. P < 0.05 was considered statistically significant.
${\bar{x}}$
± sd), median (M), minimum (min), and maximum (max) values. The normal distribution of numerical variables was evaluated with the Shapiro–Wilk test for normality. Numerical variables were compared according to weight groups with the Kruskal–Wallis test. In the Kruskal–Wallis analysis, the Dunn-Bonferroni test was used for multiple comparisons. Categorical variables were compared according to groups with Fisher’s exact test. When Fisher’s exact test yielded significant results, intergroup differences were evaluated using the two-sample z test with Bonferroni correction for each category. P < 0.05 was considered statistically significant.
Results
A total of 645 patients consisting of children and preterm and newborn babies, 152 of which were younger than 1 month of age, underwent duct closure with Piccolo between January 2016 and October 2021. The mean age was 2.2 ± 3.4 years (3 days–17 years, median 0.6 years), and the mean body weight was 10.4 ± 10.8 (0.7–83, median 7.3) kg. Of the total, 372 patients (57.6%) were female. At the narrowest point, the mean duct diameter was 1.9 ± 0.8 (1.1–4.7) mm. The mean fluoroscopy time was 6.5 ± 6 (0.9–41, median 4.3) minutes. The average device size was 4/4. The proportion of patients with each ductal anomaly type was as follows: Type A, 285 (44.1%); Type B, 9 (1.3%); Type C, 72 (11.1%); Type D, 44 (6.8%); Type E, 171 (26.5%); Type F 64 (9.9 %) (Fig. 1). Considering all patients, the overall success rate was 99.1% (Tables 1 and 2). Sixty-two patients weighed ≤ 1.5 kg, 90 patients weighed 1.5–3 kg, and the mean follow-up time was 20.3 ± 21.2 (0.1–86, median 12.4) months. The difference between groups in terms of follow-up time was not statistically significant. Of the patients weighing ≤ 1.5 kg and 1.5–3 kg, 91.9% and 81.1% underwent ductus closure through the antegrade route, respectively. In contrast, 78.1% of the patients weighing > 3 kg underwent duct closure through the retrograde route (p < 0.001). Ductus diameter was larger in groups ≤ 1.5 and 1.5– kg than in > 3 kg, and the difference was statistically significant (2.72 ± 0.8 and 2.75 ± 0.8 versus 1.66 ± 0.6, p < 0.001). Fluoroscopy time was significantly shorter in group > 3 kg than others (p < 0.001). The diameters of the devices used in groups ≤ 1.5 and 1.5–3 kg were significantly greater than those used in group > 3 kg (4.8 ± 0.6 and 5.0 ± 0.6 versus 4.4 ± 0.8, p < 0.001). The success rate of the procedure was 96.8% in ≤ 1.5 kg, 95.6% in 1.5–3 kg, and 100% in > 3 kg patients, wherein the success rate was significantly higher in group > 3 kg (p < 0.001).
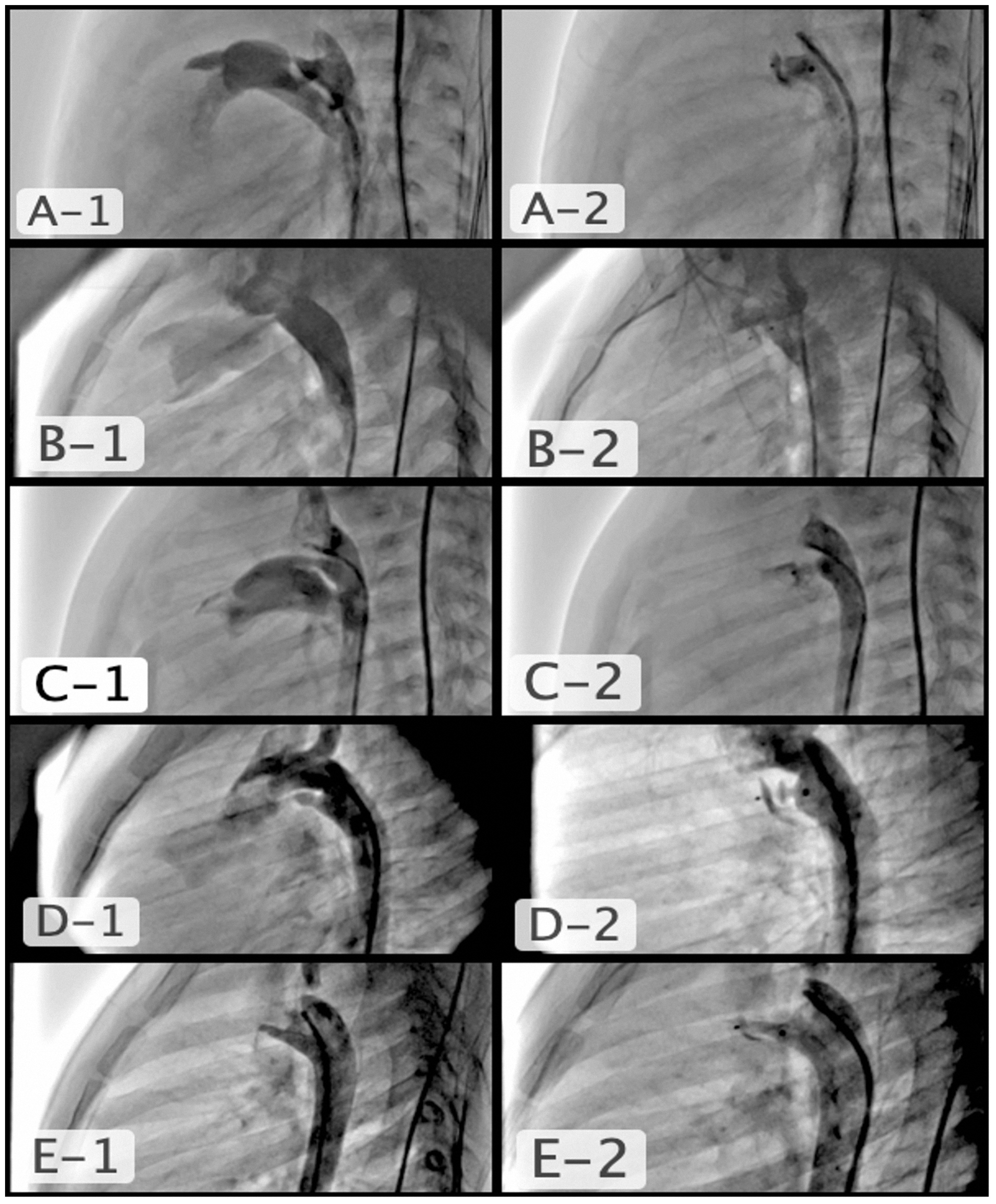
Figure 1. A1 and A2) type a conical PDA, 5-month-old baby, PDA pulmonary diameter 2.4 mm, aortic diameter 6.3 mm, length 8.2 mm, used device APO 5/4; B1 and B2) type B, aortopulmonary window type PDA, 3.5-year-old, PDA diameter 2 mm, the reason why a short device was selected, APO 5/2; C1 and C2) Tubular type C PDA, 2-month-old, PDA diameter 2.2 mm, length 10.6 mm, used device APO 4/4; D1 and D2) multiple constrictions type D PDA, 2-year-old, PDA pulmonary diameter 2 mm, a short device was selected so that it could expand before the first stenosis in order not to cause constriction in the aorta, APO 5/2; E1 and E2) elongated type E PDA, 2-year-old, PDA diameter 2.6 mm, length 12 mm, used device APO 4/6.
Table 1. Demographic and clinical data of the groups.

COA = coarctation of the aorta; LPA = left pulmonary artery; M = median; min = minimum; max = maximum; PDA = patent ductus arteriosus; sd = standard deviation.
†Kruskal–Wallis test.
‡Fisher exact test, p-values in bold are statistically significant.
a,b,cDifferent superscripts indicate statistically differences between groups in each rows. there is no statistical difference between groups with the same superscripts.
Table 2. PDA morphology and measurements according to groups.

M = Median; min = minimum, max = maximum; sd = standard deviation.
†Kruskal–Wallis test.
‡Fisher exact test, p-values in bold are statistically significant.
a,b Different superscripts indicate statistically differences between groups in each rows. There is no statistical difference between groups with the same superscripts.
There was also a difference between groups in terms of complications. Iatrogenic coarctation of the aorta was significantly more common in group ≤ 1.5 kg (p < 0.001). Iatrogenic left pulmonary artery stenosis was significantly more common in groups ≤ 1.5 and 1.5-3 than > 3 kg (p = 0.011). The distributions of ductal residual shunt and device embolisation were similar. Considering all adverse events, the percentage of patients with complications was similar in groups ≤ 1.5 and 1.5–3 kg, whereas group > 3 kg had the lowest complication rate (p < 0.001). (Table 1). Totally frequent complications (9, 14.5%) were seen in groups ≤ 1.5, such as iatrogenic different degree vessel obstruction in 6, device embolisation in 2 (one needed surgery only) and cardiac perforation in 1 patient.
As expected, the rate of Type F morphology was significantly higher in the group ≤ 1.5 kg. Type C in group 1.5–3 kg was significantly higher than the others, but because of different centres and interventionalist, some ducts might be classified such as Type C instead Type F at these group. Piccolo could be used in every type PDA’s (Table 2). Ductus aortic diameter of the group weighing > 3 kg was wider; pulmonary diameter values of the > 3 kg group were statistically narrower than the other two groups (Table 2).
Most preterm ductus was fetal type. This group had tubular ductus with no significant pulmonary stenosis (Fig. 2). As seen in the data, this group had a larger mean diameter than group > 3 kg. However, this can also be attributed to the fact that Amplatzer Ductal Occluder I was preferred in group > 3 kg patients with a large ductus, which was not included in the data analysed in this study. Similarly, patent ductus arteriosus was classified as very small and were closed with Piccolo instead of Ductal Occluder I or II in this age group (Fig. 2).
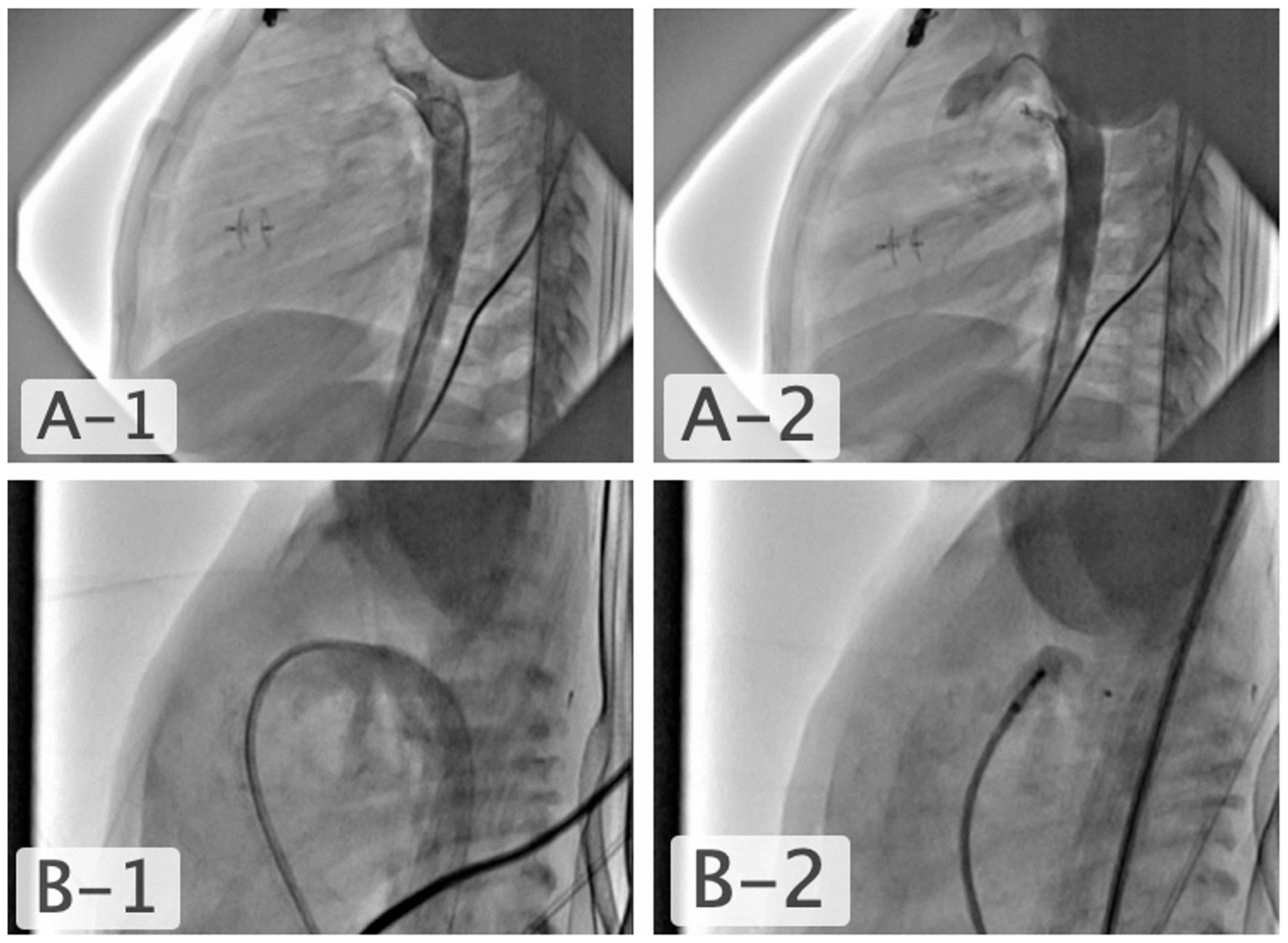
Figure 2. A1 and A2) very thin PDA, closed with APO 4/4 following transcatheter VSD closure in a 2-year-old child. B1 and B2) type F preterm PDA, the 20-day-old intubated patient weighing 1 kg had coexisting necrotising enterocolitis and bronchopulmonary dysplasia and the PDA diameter and length were measured to be 3.5 mm and 8 mm. Intraductal closure was performed with an APO 5/4.
Patients with an isolated ductus made up 87.5% of the patients. The most common comorbid heart anomaly was secundum atrial septal defect in 17, ventricular septal defect in 12, coarctation in 10, pulmonary stenosis in 8, endocardial cushion defect in 3 patients, and two patients had a history of surgical ductus ligation with a residual duct. Among patients with a haemodynamically significant shunt or stenosis, transcatheter defects closure, balloon valvuloplasty, and angioplasty were performed during the same session. Due to the advantages offered by the delivery system and discs of the Piccolo device, even in the presence of aort coarctation, it was possible to close the accompanying duct with Piccolo in many cases (Figs. 3 and 4). Occluder disks could be used in patients with coexisting coarctation without protruding into the aorta and not be the reason of increasing gradient following balloon angioplasty for coarctation. In another patient, who had a stent placed for coarctation 2o years ago, type B duct closure was safely achieved through the stent without discs protruding into the aorta (Supplemantary Figure S2).

Figure 3. A) followed by balloon dilatation of aortic coarctation in a 6-month-old patient with hypoplastic left ventricle and complete endocardial cushion defect that was treated with pulmonary banding B) the thin PDA was closed with a short APO 3/2 without protruding into the aorta during the same session.

Figure 4. 3-month-old patient with perimembranous ventricular septal defect and mitral valve stenosis A) also had aortic arch hypoplasia and a tubular PDA. B, C and D) balloon angioplasty for the coarctation and tubular PDA closure through the antegrade route with an APO 5/2 were performed during the same session without protruding into the aorta.
A total of 13 patients (2%) had device embolisation because of wrong measurement, spasm of the ductus, improper position, and physician error; 11 of which were removed with a snare catheter. One each patient from group ≤ 1.5 and 1.5–3 kg, surgical removal was performed, followed by successful ligation of the ductus. Considering our experience, retrieving the embolised device with a snare was relatively easy. This stemmed from the soft structure of the device, the ability to fit into a 5F catheter even if the device was captured and totally collapsed in seven patients from any location other than the hub, and the fact that it was quickly removed without any tripping and without being placed in the catheter in preterm babies that required the use of 4F catheters to retrieve the device partially captured in six patients at the first two groups. Unfortunately, a premature baby died due to cardiac perforation during the 0.035-inch hydrophilic guidewire manipulations at the beginning of our experience. After that we always used the floppy coronary wire. There were no other major complications or deaths. Three patients (0.4%) had mild-moderate stenosis in the left pulmonary artery, and 5 patients (8.1% in group I) had varying degrees stenosis in the descending aorta during follow-up. In two preterm newborns who developed iatrogenic coarctation of the aorta, the coarctation resolved with balloon angioplasty at 4 months and 1 year of age during follow-up (Supplemantary Figure S3). In a premature infant, who developed left pulmonary artery stenosis, the device was removed with a snare 10 days later, and reclosure was performed with shorter device (Supplemantary Figure S4). While residual shunting was observed in 1.9% of the patients on postoperative day 1, the rate of the same dropped to 0.1% at the last follow-up. All patients, except premature infants who required additional medical support and were admitted to the neonatal intensive care unit, were discharged on the day after the procedure. At the last follow-up, none of the patients had an obstruction in the descending aorta or left pulmonary artery or a significant gradient on Doppler.
Discussion
This multicentre study has shown that Piccolo Occluder could be used in all types of ducts with a diameter of 4 mm and in children of all age groups, from premature infants to adolescents. Agnoletti et al. were the first ones to report their experiences with seven children in whom Piccolo Occluder was used in 2012. Reference Agnoletti, Marini, Villar, Bordese and Gabbarini11 In a study where occluder was used off-label for ductal closure in ten premature babies weighing less than 1000 g in 2016, it was reported that the haemodynamic parameters exhibited a rapid improvement after closure and that early extubation was achieved. Reference Narin, Pamukcu, Baykan, Sunkak, Ulgey and Uzum7 Piccolo was used for ductus closure in a study consisting of 32 premature infants weighing 680–2480 g. It was reported that conservative treatment duration was reduced, and ICU stay was shorter due to the patients not requiring a left-sided thoracotomy. Reference Morville and Akhavi12 The rate of successful and effective closure was 99.1% in all age groups included in this study.
Transcatheter ductus closure requires a different approach in very low birth weight newborns, which is more challenging as it is performed on very fragile patients. On the other hand, it is possible to perform and a solid alternative to surgery, as seen in our patient group and in previous studies. Reference Rodriguez Ogando, Planelles Asensio and de la Blanca13–Reference Wang, Lin, Hsieh, Wei, Ju and Wu16 Another challenge is the fact that ducts exhibit high anatomic variability. Therefore, Amplatzer has three different devices with 27 different diameters. 17–Reference Liddy, Oslizlok and Walsh19 During ductus closure, problems related to the patients and devices other than defect size, such as high incidence of residual shunts, iatrogenic coarctation of the aorta, left pulmonary artery stenosis, increased device embolisation, and femoral artery injury should also be considered. Piccolo device with smaller retention discs can be not led to aortic or left pulmonary artery constriction with proper intraductal placement within the long and wide Type F duct in premature infants. Reference Sathanandam, Gutfinger and Morray20 In retrieval of an embolised device, the low profile and soft device structure of Piccolo enables advancing through the 4F delivery system and retrieving the device without injury to cardiac structures even if the device is not getting inside to the 4F retrieval catheter. In addition, Piccolo can even be used in the emergency treatment of cardiac perforation due to its soft structure and the low profile of the delivery system. Reference Temel, Selcuk and Baspinar21
Piccolo device offers a safe and effective alternative in closing thin ductus instead of pushable coils. Piccolo is generally preferred for relatively large diameter ducts in small babies and for small defects in older children. It can even be used in patients with coarctation due to the flat discs. In cases of aortic coarctation that require balloon angioplasty or bare-metal stents, the discs of the device can be used without protruding into the aorta as in our patients. In eligible patients with coarctation, balloon angioplasty for coarctation and ductus closure can be performed during the same session. In our series, 10 patients who had coarctation of the aorta underwent balloon angioplasty and ductus closure concurrently. To the best of our knowledge, this case series is the largest one in the literature with such a high number of patients with coarctation undergoing ductus closure.
Iatrogenic coarctation in preterm babies is related to the selection of long devices and failure to achieve intraductal placement of the device. This complication can also be managed with the transcatheter approach. Two patients developed severe iatrogenic coarctation of the aorta during follow-up and balloon angioplasty was successfully performed on them.
This study has shown that the Piccolo device is safe and effective in ductus closure in all age groups, from premature babies to adolescents. In addition, the Piccolo device used in premature babies and newborns offers advantages such as low profile, minimum risk of embolisation, and low incidence of residual shunt after closure (Supplemantary Table S1). Limitation of the study is its retrospective nature; it does not have a standard in the collection of data such as PDA measurements and follow-up frequency.
Conclusion
Amplatzer Piccolo Occluder can be considered a near-ideal device for ductus closure. It is easier to position and more stable than other devices in all types of small-medium-sized ducts. This device’s symmetry, low profile, and thinner delivery catheter enables venous or arterial approach. Considering these features, the Amplatzer Piccolo Occluder, which the FDA has also approved, would facilitate the treatment of many premature infants. Transcatheter patent ductus arteriosus closure performed in experienced centres would be a less invasive treatment option compared to surgical ligation in premature babies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951123001385.
Acknowledgements
None.
Financial supports
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation (of Turkey) and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Gaziantep University ethics committee with approved code 2021/11-24).









