INTRODUCTION
Urinary tract infections (UTIs) are frequent [Reference Foxman1] and rank first when considering healthcare-associated infections [Reference Burton2]. While community-acquired UTIs and healthcare-associated UTIs are two distinct entities with different risk factors, both are associated with morbidity and important economic consequences [Reference Foxman1, Reference Burton2]. When occurring in hospital, UTIs are associated with an indwelling urinary catheter in 75% of cases, making catheter-associated UTI (CAUTI) prevention a priority [Reference Burton2]. In the intensive care unit (ICU), 95% of UTIs are CAUTIs and despite guidelines and efforts to reduce CAUTI incidence, they remain the second most important healthcare-associated infection in critically ill patients [Reference Burton2, Reference Leblebicioglu3].
The diagnosis of UTI is based on a combination of clinical and microbiological parameters [Reference Foxman1, Reference Horan, Andrus and Dudeck4]. In critically ill patients, the diagnosis can be particularly challenging, given the presence of impaired consciousness and/or of systemic inflammation. Therefore, in ICU, asymptomatic bacteriuria is often considered the same as symptomatic UTI and treated [Reference Cope5]. In addition, antibiotic therapy continuously impacts on microbiological ecology contributing to the emergence of pathogens including Candida as a source of bladder colonization or infection [Reference Singla6].
Therefore, developing a better understanding of the modern epidemiology of bacteriuria and candiduria diagnosed in ICU should play a key role in improving management. In addition, recent studies focusing on critically ill patients did not report the rate of ICU-acquired positive urine culture associated with blood stream infection (BSI) [Reference Burton2, Reference van der Kooi7]. Updating the findings of older reports which found that BSI related to ICU-acquired UTI was rare (around 1–3%) [Reference Bagshaw and Laupland8, Reference Chang9] is important.
Accordingly, we conducted a retrospective cohort analysis of the epidemiology of bacteriuria and candiduria in a large cohort of critically ill patients.
SUBJECTS AND METHODS
Study design
We conducted a retrospective study over a 6-year period at a university affiliated hospital (The Austin Hospital, Melbourne, Australia). All patients aged >16 years, who had at least one positive urine culture obtained in ICU between January 2006 and December 2011, were enrolled. The 20-bed ICU of The Austin Hospital is the liver transplantation and spinal trauma referral service for Victoria and Tasmania, and is a referral centre for complex aortic surgery. The study was approved by the local human research ethics committee (H2013/05071).
Patients and data
Patient characteristics including, age, gender, admission diagnosis and comorbidities were extracted from the Australian New Zealand Intensive Care Society (ANZICS) Adult Patient Database (APD). The ICU and hospital admission and discharge dates were also collected. The hospital admission sources were grouped as following: home and other hospital; the ICU admission sources were grouped as following: emergency department, ward, operating room and other hospital. Acute Physiology and Chronic Health Evaluation (APACHE) III score was used to evaluate ICU admission severity.
Microbiology data
Microbiological data were retrieved from the microbiology laboratory records (Kestral system, www.kestral.com.au, Australia). In ICU, urine collected for culture is obtained aseptically through an indwelling urinary catheter. Identification tests were performed by the microbiology laboratory using standard culture and bacteriological testing.
Bacteriuria and candiduria episode definition and classification
Individual bacteriuria and candiduria were defined by the presence of a positive urine culture collected in ICU, with no more than two pathogens, not isolated previously, and at ⩾105 colony-forming units (c.f.u.)/ml [Reference Horan, Andrus and Dudeck4]. Because of the absence of clinical data and other biological parameters on the day of the urine culture, any attempt to distinguish symptomatic and asymptomatic bacteriuria/candiduria was not possible and all positive urine cultures were expressed as bacteriuria or candiduria [Reference Horan, Andrus and Dudeck4].
Community-acquired bacteriuria/candiduria were defined as positive urine cultures occurring within 48 h of hospital admission in patients admitted from home. In all other cases, positive urine cultures were classified as healthcare-associated bacteriuria/candiduria. They included: (i) ward-acquired bacteriuria/candiduria when collected within the first 48 h of ICU stay and (ii) ICU-acquired bacteriuria/candiduria when collected after 48 h of ICU stay. Urine cultures are usually performed when the patient has one of the following findings: fever, high white count cells or haemodynamic deterioration.
Patients who had candiduria and bacteriuria during the same ICU admission were excluded from the analysis comparing the two conditions.
An ICU-acquired positive urine culture was considered to be associated with a BSI if there was a concurrent or subsequently positive blood culture with the same organism (same species) within a 14-day period [Reference Greene10].
The rates of ICU-acquired positive urine cultures/1000 ICU days were calculated. The study period was split into two time periods: from 2006 to 2008 and from 2009 to 2011 to analyse temporal trends.
Statistical analysis
Variables were assessed for normality. Baseline comparisons were performed using Fisher's exact tests and reported as proportions. Continuous normally distributed variables were compared using Student t tests and reported as means±standard deviation, while non-normally distributed data were compared using Wilcoxon rank-sum tests and reported as medians (interquartile range; IQR). Temporal trends were assessed by comparing the data collected in the first half with those in the second half of the study period by means of Student's t test or Fisher's exact test. All analyses were performed by using JMP v. 8.0.2 (SAS Institute, USA). A two-sided P value of <0·05 was considered to be statistically significant. All ICU-acquired positive urine cultures were considered for the descriptive analysis of pathogens and calculation of incidence. Only the ICU admission with the first bacteriuria or candiduria was considered when comparing patients with community- and ICU-positive urine cultures or ward- and ICU-acquired positive urine cultures.
RESULTS
Between January 2006 and December 2011, 10 034 patients were admitted to ICU for a total of 12 244 ICU admissions. Four hundred and forty-four unique bacteriuria/candiduria episodes were diagnosed in 406 patients. These were community-acquired in 67 (15%) cases and healthcare-associated in 377 (85%) cases. One hundred and fifty-six positive urine cultures were classified as ward-acquired and 221 bacteriuria/candiduria as ICU-acquired leading to an incidence of 6·4/1000 ICU days (Fig. 1).
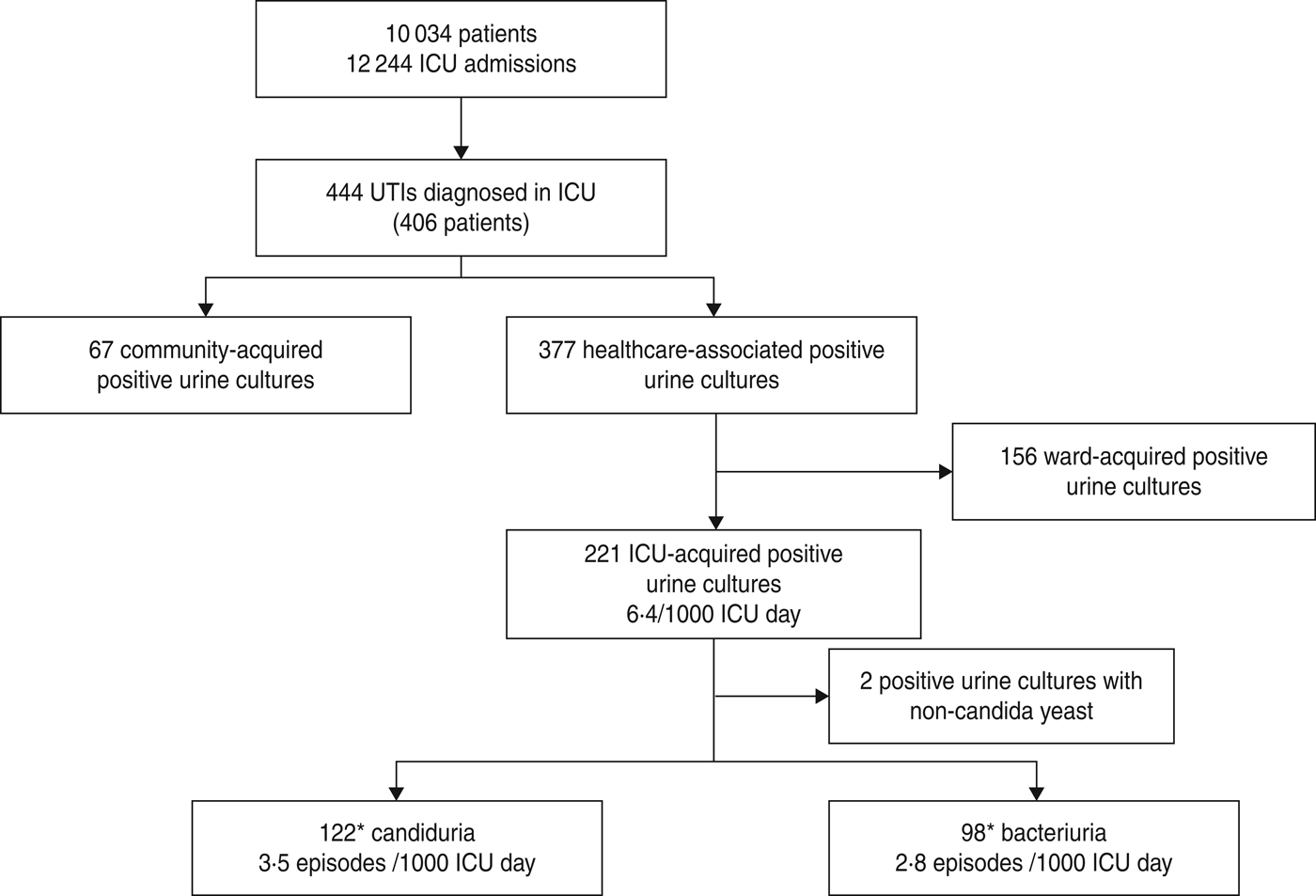
Fig. 1. Epidemiology of positive urine culture diagnosed in intensive care unit (ICU), based on patient's admission source and pathogen type. * One episode was classified as both ICU-acquired candiduria and ICU-acquired bacteriuria because of concomitant candiduria and bacteriuria.
Characteristics of patients with community- and ICU-acquired bacteriuria/candiduria
Table 1 shows the characteristics of the 65 patients who had community-acquired positive urine cultures, and those of the 205 patients with ICU-acquired positive urine cultures (two patients who had both community- and ICU-acquired positive urine cultures were excluded from this comparison). Bacteriuria/candiduria occurred predominantly in females (45/65, 69%) in the community-acquired group, and 136/205 (66%) in the ICU-acquired group. There were no significant differences between both groups regarding age, or illness severity. The ICU and hospital length of stay (LOS), both overall and after the positive urine culture, were significantly shorter in patients who had community-acquired bacteriuria/candiduria [median hospital LOS 11·2 days (IQR 5·4-21·9) vs. 33 days (IQR 19·5-56·8), P < 0·0001; hospital LOS after positive urine culture I 10 days (IQR 4·5-21·5) vs. 22 days (IQR 11·41·5), P < 0·0001].
Table 1. Comparison of characteristics of patients with community- and ICU-acquired positive urine culture diagnosed in ICU
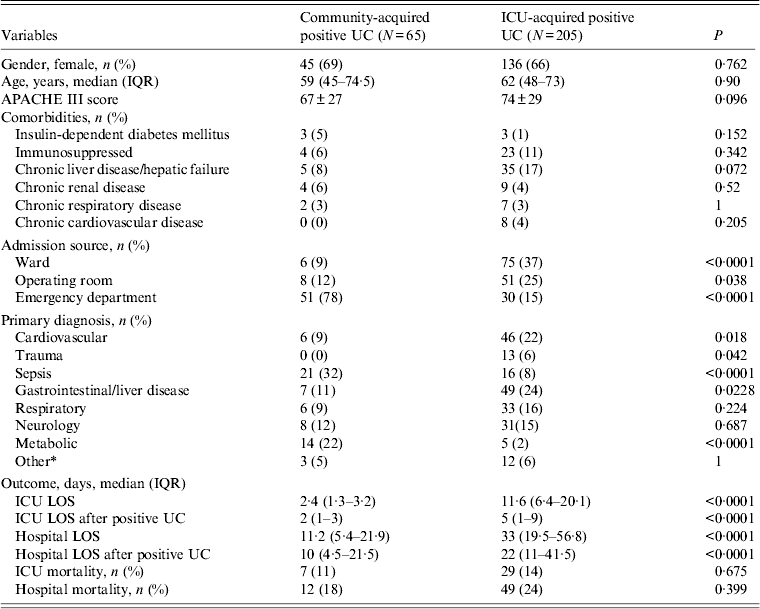
ICU, Intensive care unit; IQR, interquartile range; UC, urine culture; APACHE III score, Acute Physiology and Chronic Health Evaluation III score; LOS, length of stay.
* Other includes renal disorder, haematology disorder.
Microbiology characteristics of community- and ICU-acquired bacteriuria/candiduria
Pathogens responsible for community- and ICU-acquired positive urine cultures are given in Table 2. Seventy-three pathogens were isolated from community-acquired bacteriuria/candiduria. They were Enterobacteriaceae in 64% of cases with Escherichia coli as the most frequent bacteria (32/64 bacteria isolated, 50%) (Table 2). Two hundred and thirty-six pathogens were isolated from ICU-acquired bacteriuria/candiduria. They included Enterobacteriaceae in 24% of cases with E. coli as the most common bacteria (31/107 bacteria isolated, 29%). However, Candida spp. were the most common group of microorganisms isolated overall (55%), with C. albicans in 65% (84/129) of cases. Finally, six (8·9%) of community-acquired positive urine cultures and 18 (8·1%) ICU-acquired positive urine cultures were polymicrobial.
Table 2. Pathogens responsible for positive urine cultures collected in ICU
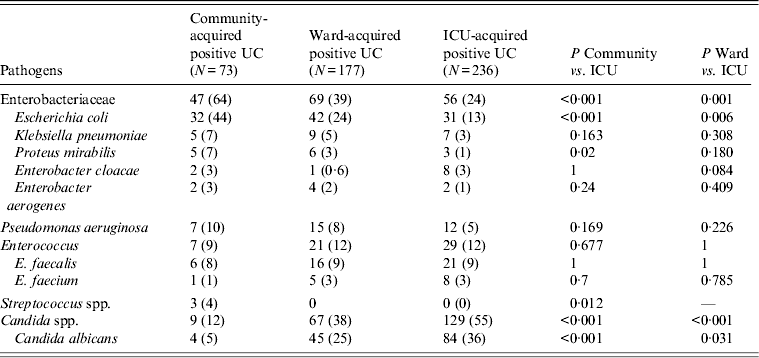
ICU, Intensive care unit; UC, urine culture.
Values given are n (%).
Ward-acquired and ICU-acquired positive urine culture
Patients who had an ICU-acquired positive urine culture were significantly younger than those with a ward-acquired positive urine culture (62 vs. 68 years, P = 0·005). They also suffered more often from chronic liver disease or hepatic failure (17% vs. 8%, P = 0·032). The ICU and hospital LOS were, both overall and after the positive urine culture, longer in the group of patients with ICU-acquired bacteriuria/candiduria (Table 3). Microbiology patterns were also significantly different between both groups. Enterobacteriaceae and E. coli were isolated more often in the group of patients with ward-acquired positive urine culture (39% vs. 24%, P = 0·001 and 24% vs. 13%, P = 0·006, respectively), while Candida was less frequently isolated in the group of patients with ward-acquired positive urine culture (55% vs. 38%, P < 0·001).
Table 3. Comparison of characteristics of patients with ward- and ICU-acquired positive urine culture diagnosed in ICU
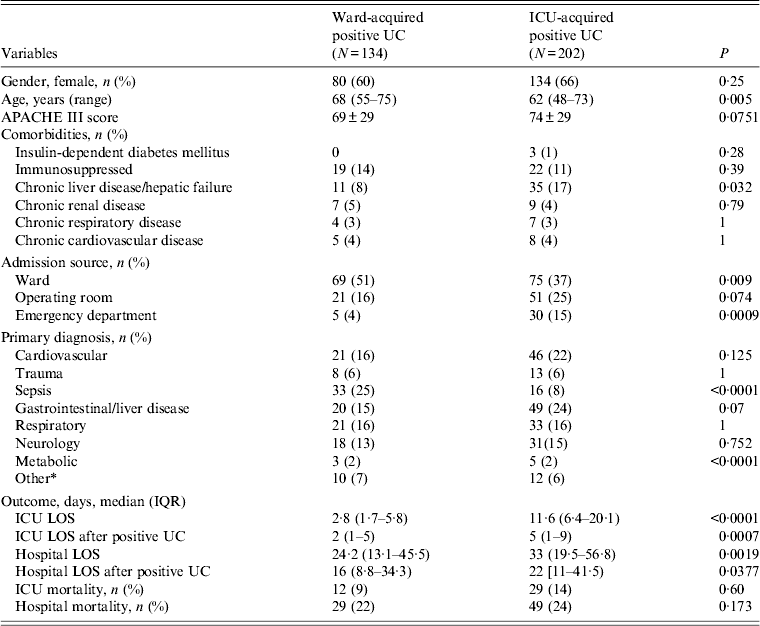
ICU, Intensive care unit; IQR, interquartile range; UC, urine culture; APACHE III score, Acute Physiology and Chronic Health Evaluation III score; LOS, length of stay.
* Other includes renal disorder, haematology disorder.
ICU-acquired candiduria and ICU-acquired bacteriuria
More than half of the ICU-acquired positive urine cultures were due to Candida spp. (3·5 episodes/1000 ICU days), while ICU-acquired bacteria occurred with an incidence of 2·8 episodes/1000 ICU days (Fig. 1).
Of the 205 patients with ICU-acquired positive urine cultures, two patients had non-Candida yeasts isolated alone and six had candiduria and bacteriuria in the same ICU admission. During their ICU admission with the first positive urine cultrure episode, the 110 patients developing ICU-acquired candiduria had significantly higher APACHE III scores on admission than the 87 patients developing only ICU-acquired bacteriuria (79 ± 25 vs. 66 ± 31, P = 0·0015) (Table 4). They also had a significantly higher incidence of chronic liver disease and/or a hepatic failure (23% vs. 8%, P = 0·0062) (Table 4) and were in hospital for a longer period of time compared to patients who had ICU-acquired bacteriuria [median 12 days (IQR 6-17) vs. 6 days (IQR 3-15), P = 0·0016] (Table 4). Patients with candiduria had a longer ICU LOS but not a longer hospital LOS after the positive urine culture. Figure 2 shows the timing of occurrence of ICU-acquired candiduria and bacteriuria. There was no difference in mortality between patient groups (Table 4).
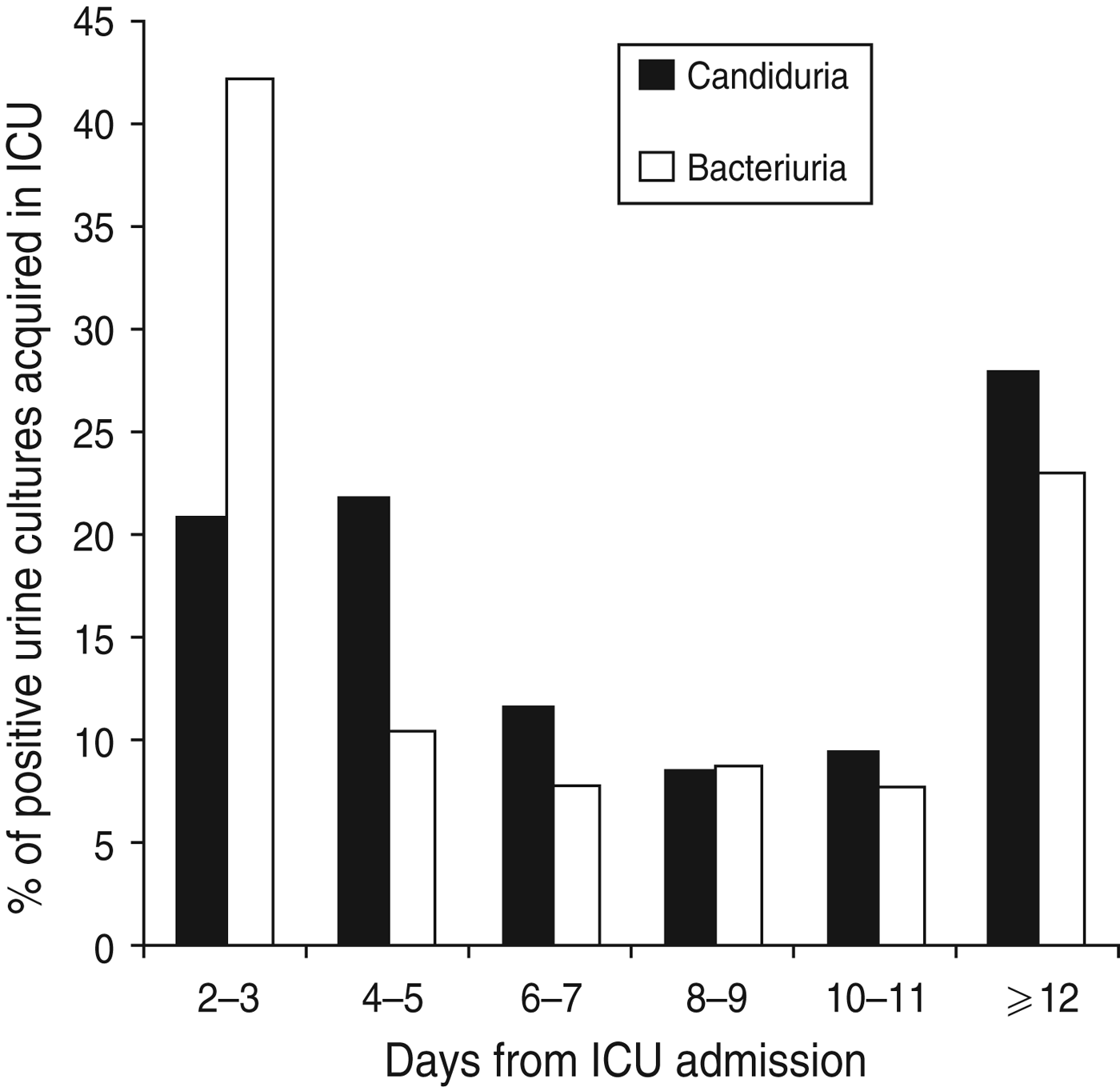
Fig. 2. Timing of occurrence of intensive care unit (ICU)-acquired bacteriuria and candiduria over ICU stay.
Table 4. Characteristics of patients with ICU-acquired urinary tract infections and comparison between patients with ICU-acquired candiduria and with ICU-acquired bacteriuria
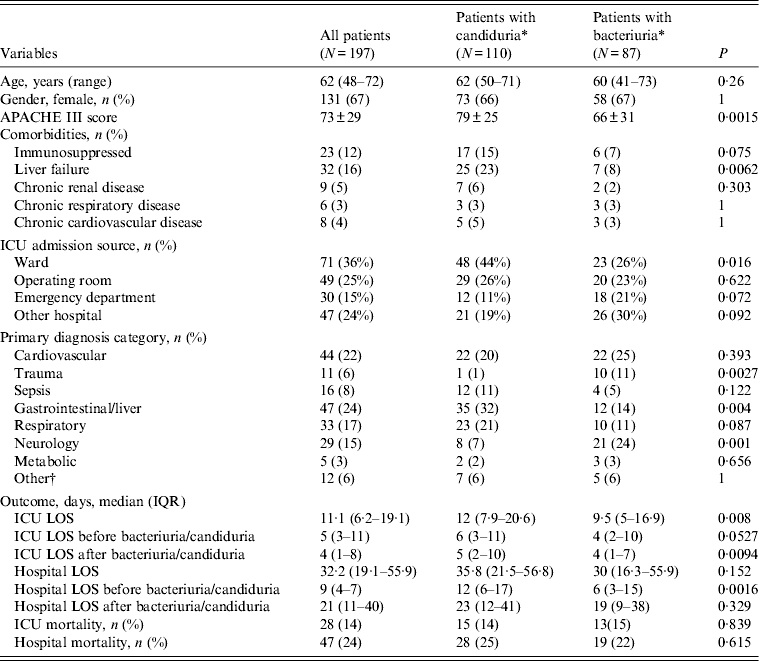
ICU, Intensive care unit; IQR, interquartile range; APACHE III score, Acute Physiology and Chronic Health Evaluation III score; LOS, length of stay.
* Patients with a same culture positive with bacteriuria and candiduria were excluded.
† Other includes renal disorder, haematology disorder.
Only the first episode was considered.
ICU-acquired bacteriuria-/candiduria-associated BSI
ICU-acquired positive urine cultures were associated with BSI in six cases leading to a rate of 0·17 positive urine culture-associated BSI/1000 ICU days. They occurred in six patients, including three who were immunosuppressed. They were due to Enteroccocus faecium in two cases and Candida in four cases (three with C. albicans and one with C. glabrata). ICU mortality was 33% in patients with positive urine culture associated with BSI and 14% in patients with positive urine culture without BSI (P = 0·20); while hospital mortality was significantly higher when a positive urine culture was associated with BSI (67% vs. 23%, P = 0·023).
Temporal trends
The annual mean rate of ICU-acquired positive urine culture/1000 ICU days remained stable over time (6·1 ± 0·8/1000 ICU days between 2006 and 2008, and 6·6 ± 0·9/1000 ICU days between 2009 and 2011, P = 0·56). There was also no significant change in the rate of ICU-acquired candiduria (3·2 ± 0·9 episodes/1000 ICU days vs. 3·8 ± 0·9 episodes/1000 ICU days, P = 0·86) or ICU-acquired bacteriuria (3·0 ± 0·26 and 2·7 ± 0·1 episodes/1000 ICU days, P = 0·19) (Fig. 3). In the first study period, C. albicans was responsible for 71% of candiduria and for 60% in the second period of the study (P = 0·19) with an increased incidence of C. glabrata. The three most frequently isolated bacteria from ICU-acquired positive urine culture were E. coli, E. faecalis and Pseudomonas aeruginosa; this remained the same over the time.

Fig. 3. Temporal trend of intensive care unit (ICU)-acquired positive urine culture.
DISCUSSION
We studied the epidemiology of positive urine cultures diagnosed in ICU, highlighting the heterogeneity of this entity in terms of patient sources, admission diagnosis category and causative organisms. In our tertiary referral ICU, Candida spp. was a more frequent cause of ICU-acquired positive urine culture than bacteria and a relative common cause of positive urine culture-associated BSI. These findings suggest the need to focus on surveillance for and prevention of urinary tract colonization with Candida in ICU patients and on the implementation of methods to rapidly identify patients, who become colonized.
Community-acquired positive urine cultures
Patients with community-acquired positive urine culture were predominantly female in keeping with other findings [Reference Linhares11, Reference Marcus12]. Contrary to what is usually reported, we found E. coli was responsible for less than half of the community-acquired positive urine cultures, while Pseudomonas spp. and Candida were more associated with community-acquired positive urine cultures. This trend has also been recently reported in a study of 18 797 community-acquired UTIs, where Pseudomonas was isolated in 5% of cases [Reference Linhares11]. Our results may be explained by the fact that patients with community-acquired positive urine cultures had comorbidities and a history of healthcare facility stay that other patients with community-acquired positive urine culture usually do not typically have. It is well described that healthcare facility stay and comorbidities impact on microbiology patterns. A paediatric study comparing E. coli and non-E. coli community-acquired UTIs found that patients with non E. coli UTIs received antibiotic therapy more often prior to the UTI episode than those with E. coli UTIs [Reference Marcus13].
Ward-acquired positive urine cultures
Comparison of microbiology patterns between ICU- and ward-acquired positive urine cultures showed that healthcare-associated pathogens are more commonly isolated in ICU than in the ward, suggesting a higher exposure to antibiotics in ICU. Our results are in accordance with those of Lewis et al. who reported that Candida spp. were more frequently isolated in ICU- than in non-ICU-acquired UTIs [Reference Lewis14].
ICU-acquired candiduria and bacteriuria
The incidence of ICU-acquired positive urine culture in our population was 6·4/1000 ICU days. This is consistent with the published literature. The incidence of CAUTIs in critically ill patients reported in the literature is highly variable, ranging from 2·8 to 11·3 episodes/1000 ICU days [Reference Burton2, Reference Chant15]. This high variability is secondary to high variability in diagnostic criteria but also in study population [Reference Burton2, Reference Bagshaw and Laupland8]. Laupland et al. reported higher rates of 9·6 and 11·3/1000 ICU days in two retrospective ICU studies of 4465 and 1981 patients with a similar case definition [Reference Laupland16, Reference Laupland17] while Burton et al. found lower rates of CAUTIs fluctuating between 2·7 and 4·4/1000 ICU days in a study of 59 255 CAUTIs in ICU patients [Reference Burton2]. Current literature suggests patients developing CAUTI are commonly females aged >60 years with stays exceeding 7 days [Reference van der Kooi7, Reference Lewis14, Reference Laupland16], this is also suggested by our findings.
In keeping with previous publications, Gram-negative bacilli were the most common bacterial pathogens isolated in ICU-acquired positive urine culture [Reference Chant15], with E. coli remaining one of the most common [Reference Burton2, Reference Lewis14]. Candida spp., however, were responsible for 55% of the positive urine cultures in our population and this is potentially explained by the presence of a unique cohort of liver failure patients. However, liver failure does not appear as a common predisposing factor for candiduria [Reference Sobel18]. A meta-analysis of 11 studies and 2745 ICU patients with CAUTI suggested that a fungal source may account for up to 34% of such infections [Reference Chant15]. In their large retrospective analysis of 59 255 CAUTIs over three decades, Burton et al. found that C. albicans was the most common isolated microorganism; however, it remained responsible for <20% of infections by case definition [Reference Burton2]. In reports with only microbiology criteria for CAUTI definition, the percentage reported was between 20% and 29% suggesting that the difference observed cannot be explained by difference in diagnostic criteria [Reference Laupland16, Reference Laupland17, Reference Clec'h19].
With a rate of 3·5 episodes of ICU-acquired candiduria/1000 ICU days, our results are comparable to those of Yang et al. These authors recently reported rates of around four episodes of candiduria/1000 ICU days in a retrospective study of 186 critically ill patients with ICU-acquired fungal infections, including 89 UTIs based on the same and unique microbiology definition criteria [Reference Yang20]. In this report, there was also an association between Candida infection and patient severity (APACHE III score) [Reference Yang20]. Finally, the most common urinary fungal isolates were C. albicans, C. glabrata and C. tropicalis, in accord with our findings [Reference Kauffman21].
ICU-acquired positive urine cultures associated with BSI
BSIs are rarely associated with CAUTIs [Reference Bagshaw and Laupland8, Reference Saint22]. In our study, they occurred in 2·7% of positive urine cultures (0·17 cases associated BSI/1000 ICU days). Laupland et al. reported four cases (0·1 CAUTI-related BSI/1000 ICU days) in a 3-year retrospective study including 356 CAUTIs [Reference Laupland16]. Immunosuppression is a risk factor for CAUTI-related BSI [Reference Greene10, Reference Saint22]. Enterococcus and species belonging to the genus Enterobacteriaceae are usually the most common pathogens responsible for CAUTI-related BSIs [Reference Chang9, Reference Greene10, Reference Saint22]. Nonetheless, a recent case-control study of 320 cases of CAUTI-related BSIs reported Candida as the second most common microorganisms isolated [Reference Chang9]. This study also highlights the high risk of CAUTI-related BSIs due to Candida [Reference Chang9] suggesting that Candida spp. are an important agent of infection [Reference Kauffman21].
Implication of study findings
Microbiological findings for community-acquired positive urine cultures support the need for further investigations to determine if non-E. coli community-acquired bacteriuria represents a true community-acquired or a healthcare-associated positive urine culture.
Our results suggest that candiduria occurs in female patients with liver failure in hospital for >10 days and in ICU for >6 days. They also support an association between candiduria and increased patient morbidity. However, candiduria is also likely to be a marker of patient severity and it has been reported that treatment of candiduria did not avoid recurrence and did not impact on patient outcome [Reference Sobel23, Reference Revankar24]. Further studies are required to evaluate the risk of developing fungaemia after candiduria, as we were not able to assess whether candiduria followed or preceded fungaemia.
There are several limitations to our study. This is a retrospective single-centre study and our results may have limited external validity. However, our hospital has all of the features of a typical tertiary referral academic centre in a developed country. We only examined patients with positive urine culture where the cultures had been taken in the ICU. An exhaustive analysis of risk factors for candiduria would require information regarding total parenteral nutrition, antibiotic and antifungal exposure and invasive procedure exposure, which may be important confounders and all likely to contribute to the incidence of candiduria [Reference Yang20]. We were unable to differentiate asymptomatic and symptomatic bacteriuria; however, standard UTI criteria are not relevant to sedated ICU patients [Reference Conway and Larson25]. A more robust measure of the incidence of ICU-acquired positive urine cultures may have been rates calculated per catheter day. However, this information was not available in our ICU.
In conclusion, our study describes the epidemiology of bacteriuria and candiduria in critically ill patients, based on patient source and pathogens isolated. Candida emerged as a key pathogen for ICU-acquired positive urine cultures and positive urine culture-associated BSI in ICU. Prospective interventional studies are warranted in high-risk patients, which focus on surveillance, prevention and management of candiduria when deemed appropriate.
DECLARATION OF INTEREST
None.










