Depression as a global health problem
According to the World Health Organization, depression is the ‘leading course of disability worldwide’ and ‘a major contributor to the overall global burden of disease’, with more than 264 million people of all ages with depressive disorders.1 Affecting performance at school or work, as well as relationships and family constellations, depression threatens not only the global living environment of individuals, but of society as a whole.Reference Cassano and Fava2
Depressive disorders in adolescence
Depressive disorders are common mental health problems during adolescence. In a survey conducted in the USA, a lifetime prevalence of about 12% was found for the adolescent sample, and prevalence nearly doubled from early (13/14 years: 8.4%) to late adolescence (17/18 years: 15.4%).Reference Merikangas, He, Burstein, Swanson, Avenevoli and Cui3 Additionally, the number of adolescents with depressive symptoms is even higher when considering the high number of adolescents with subthreshold depression, with a lifetime prevalence of up to 20% at the age of 18 years.Reference Bertha and Balázs4
Depressive disorders during adolescence are associated with more burdensome circumstances in adulthood, including mental health problems, suicidal ideation or attempts, reduced educational attainment and economic status, lower relationship quality and higher levels of conflicts and violence.Reference McLeod, Horwood and Fergusson5 This underlines the importance of a deeper understanding of the aetiology and severity of depressive disorder.
Sleep disturbance in patients with depressive disorder
Sleep disturbance constitutes an important symptom of depressive disorders, and it gains even greater importance when considering that it may increase the risk of suicidal behaviour among adolescents.Reference Goldstein, Bridge and Brent6 Delayed sleep onset and decreased sleep efficiency, combined with changes in rapid eye movement (e.g. shorter latency and increased activity, density and amount of rapid eye movement sleep) and slow-wave sleep (e.g. decreased sleep amount), are frequent findings from studies assessing sleep in adults with depression.Reference Rao7 Regarding paediatric depression, findings are far more heterogeneous. In his review dealing with sleep disturbance in paediatric depression, Rao considered 24 electroencephalogram studies conducted in children and adolescents.Reference Rao7 A prolonged sleep latency was found in eight of these studies, and 15 studies found no differences. A decrease in sleep efficiency was found in three of the studies, and 19 studies found no differences.Reference Rao7 As sleep underlies multifarious influences in the context of development, the heterogenous study situation could reflect the complexity of sleep quality and its components during the developmental period.Reference Rao7 Examination of sleep latency and efficiency via accelerometry, as conducted in this study, has been shown to be a promising approach in previous research.Reference Lemola, Ledermann and Friedman8,Reference Meltzer, Short, Booster, Gradisar, Marco and Wolfson9
In addition to objective measures of sleep quality, the burden of impaired subjective sleep quality and sleep complaints caused by depression should be considered. Many studies in adults with depressive disorders have assessed subjective sleep quality, and findings show that over 80% of patients report sleep difficulties.Reference McCall, Reboussin and Cohen10 Previous research showed that similar impairments can be found in adolescents with depressive disorders. Considering only subjective sleep assessments without objective sleep data, Gupta et al found impaired sleep quality, delayed sleep onset, shorter sleep duration, and a correlation of sleep quality with severity of depressive symptoms.Reference Gupta, Sagar and Mehta11 These abovementioned impairments in subjective as well as objective sleep quality may lead to serious mental burden and an increased stress level in everyday life for many adolescents with depression; therefore, both measurements are important to consider.
Cortisol
A hormone that plays a key role in the experience and management of stress is the glucocorticoid hormone cortisol. Its secretion from the adrenal cortexReference Fries, Dettenborn and Kirschbaum12 depends on stimulation by adrenocorticotropin released by the anterior pituitary gland (hypothalamus–pituitary–adrenal (HPA) axis activation), and follows a characteristic diurnal rhythm with a peak in the early morning.Reference Fries, Dettenborn and Kirschbaum12 Activation of the HPA axis and the ability to adjust cortisol release is an important contributor for adapting to stressful life events, and providing energy and physical resources to cope with the demanding situation.Reference Adam and Kumari13 Chronic activation, however, is linked to various somatic and mental health problems.Reference Adam and Kumari13,Reference Schmidt, Laessle and Hellhammer14 In our study, we focus on the strength of the cortisol peak within 45 min after awakening, known as the cortisol awakening response (CAR).Reference Adam and Kumari13 Characteristics of the CAR include a precipitous increase after awakening, a maximum within about 30 min after awakening and a subsequent decline.Reference Fries, Dettenborn and Kirschbaum12 CAR constitutes a ‘reliable biological marker for adrenocortical activity’.Reference Pruessner, Wolf, Hellhammer, Buske-Kirschbaum, von Auer and Jobst15 An increased as well as a decreased CAR is associated with stress and multifarious health problems, such as autoimmune, allergic and psychiatric illnesses.Reference Adam and Kumari13,Reference Stroud, Vrshek-Shallhorn, Norkett and Doane16 Several previous studies examined changes in the CAR in differently structured samples of adults with depressive disorders. Bhagwagar et alReference Bhagwagar, Hafizi and Cowen17 observed an increased CAR in adult patients with depressive disorders; Vreeburg et alReference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck18 found similar results in patients with remitted and current depressive symptoms. Additionally, Stroud et alReference Stroud, Vrshek-Shallhorn, Norkett and Doane16 examined an interaction between larger CAR and a greater extent of depressive symptoms, whereas Huber et alReference Huber, Issa, Schik and Wolf19 found an attenuated CAR in psychotherapy in-patients with depression. In adults with subclinical depressive symptoms, an attenuation of the CAR was found by Mangold et al.Reference Mangold, Marino and Javors20 According to Elder et al,Reference Elder, Wetherell, Barclay and Ellis21 the use of antidepressant medication may be a contributing factor to the inconsistency of findings, and so we include only participants who are not taking antidepressant medications. Regarding our study cohort, previous research with adolescent study samples is of particular interest. In young girls with depressive disorders, Schmidt et alReference Schmidt, Laessle and Hellhammer14 found a significantly higher CAR. However, this study did not exclude adolescents on antidepressant medications; therefore, our study design with a medication-free sample adds important value to the current study situation.
Alpha-amylase
Another measurement for the activation of physiological stress systems is the enzyme alpha-amylase. It is secreted by the sympathetic and parasympathetic innervated salivary glands, and constitutes an important biomarker of autonomic nervous system activity with a sensitive responsiveness to acute stress situations.Reference Nater, La Marca, Florin, Moses, Langhans and Koller22 Examining alpha-amylase levels of adult patients with depression, recent studies found elevated salivary alpha-amylase levels after awakening, and provided evidence that alpha-amylase may be a promising biomarker for depressive disorders.Reference Bauduin, van Noorden, van der Werff, de Leeuw, van Hemert and van der Wee23 Regarding paediatric depression, very few studies have examined alpha-amylase levels in children and adolescents with depression.Reference Jezova, Trebaticka, Buzgoova, Durackova and Hlavacova24 To our knowledge, no study has focused on the reactivity of alpha-amylase with various measurements after awakening in adolescent patients.
The examination of alpha-amylase provides further information regarding the stress system dysregulation compared with an exclusive assessment of cortisol,Reference Ali and Pruessner25 which makes a parallel measurement of these two biomarker a useful approach.
Interrelation between sleep, depressive symptoms and psychophysiological correlates
When examining the link between sleep quality, depressive symptoms and psychophysiological correlates, the interrelations are multifactorial and the direction is partially unclear. Fang et al found that the association between sleep and depressive symptoms in adult patients with depression is bidirectional, with sleep impairment being a comorbidity as well as a prodromal sign of a depressive disorder.Reference Fang, Tu, Sheng and Shao26 In adolescent patients, previous studies reported a connection between sleep problems and later depressive symptoms,Reference Kuhlman, Chiang, Bower, Irwin, Seeman and McCreath27 but prior research also identified that higher severity of depressive symptoms was associated with poorer sleep quality.Reference Raniti, Allen, Schwartz, Waloszek, Byrne and Woods28
There are also indications that sleep and stress biomarkers are linked bidirectionally. A hyperactivity of the HPA axis can influence sleep architecture and decrease sleep quality, but sleep impairments also negatively affect functioning of the HPA axis.Reference Buckley and Schatzberg29 A previous study observed a positive association between sleep duration and levels of cortisol, but no association between subjective sleep quality and cortisol levels at awakening, whereas levels of alpha-amylase at awakening were not significantly associated with sleep duration, but a worse subjective sleep quality was associated with higher alpha-amylase levels at awakening.Reference van Lenten and Doane30
Much research was performed examining the relationship between cortisol and depressive symptoms in adolescents and adults. In this context, the release of cortisol is considered a heritable trait,Reference Dedovic and Ngiam31 with a connection to currentReference Schmidt, Laessle and Hellhammer14 and futureReference Stroud, Vrshek-Shallhorn, Norkett and Doane16 depressive symptoms. Referring to alpha-amylase, prior research found elevated alpha-amylase levels at awakening in adult patients with depression,Reference Bauduin, van Noorden, van der Werff, de Leeuw, van Hemert and van der Wee23 but first investigations in an adolescent sample did not confirm this alpha-amylase morning elevation in adolescent patients with depression.Reference Jezova, Trebaticka, Buzgoova, Durackova and Hlavacova24
Implications for the planned study
According to Nater et al, the simultaneous examination of HPA axis indicators (cortisol) and biomarkers of the autonomic nervous system (alpha-amylase) is a recommended approach, providing further information about psychobiological stress responses.Reference Nater, La Marca, Florin, Moses, Langhans and Koller22 By repeatedly analysing cortisol as well as alpha-amylase levels after awakening in adolescents with depression and healthy controls, we are able to assess neuroendocrinological biomarkers that may play a key role in many depressive symptoms, including sleep impairments. Based on the secretion mechanism and physiological compartment, we do not expect a correlation between cortisol and alpha-amylase secretion.
Additionally, our study design enables us to examine sleep and psychophysiological correlates as close to reality as possible. Examining sleep in participants’ home environmentsReference McCall, Reboussin and Cohen10 and the simple and non-invasive wearing of a watch-like accelerometer barely influences participants’ natural sleeping habits,Reference Meltzer, Short, Booster, Gradisar, Marco and Wolfson9 and makes a realistic assessment of subjective as well as objective sleep quality possible. Together with saliva sampling in a familiar environment,Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner and Wüst32 these ambulatory assessment methods lead to a high ecological validity of the study findings.
Study aim
Investigating the relationship between sleep quality and depressive symptoms, our study's goal is to compare objective measurements and subjective assessments of sleep quality between adolescents with depressive disorders and healthy peers. Another focus of our study lies in the examination of salivary cortisol and alpha-amylase releasing patterns after awakening as a potentially correlated factor in altered sleeping patterns.
Hypotheses
We hypothesise that adolescents with depressive disorders will show a lower objective sleep quality with lower sleep efficiency, longer onset latency, more frequent awakenings and longer wakefulness after sleep onset (WASO) than healthy controls. Based on previous studies, we expect the subjective sleep quality of participants with depression to be considerably lower than that of the control group (less satisfaction with sleep, worse mood after awakening, lower feeling of recreation after awakening, impression of frequent awakenings during the night and impaired subjective quality of dreams). Additionally, in accordance with Schmidt et alReference Schmidt, Laessle and Hellhammer14 and Bauduin et al,Reference Bauduin, van Noorden, van der Werff, de Leeuw, van Hemert and van der Wee23 we hypothesise an increased CAR and elevated alpha-amylase levels after awakening in the index group compared with the healthy control group.
Method
Study design
This is a non-interventional and parallelised study with a matched control group. The study was conducted monocentrally at the out-patient and day patient department of the Clinic of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy at the University of Regensburg, Germany. We choose the out-patient and day patient department to reduce sleep disturbance from the hospital environment or shared rooms expected for those patients admitted to hospital. By choosing a patient cohort that sleeps in their usual surroundings without external influences, we aim to focus on changes in sleep quality that can be attributed to the depressive disorder.
The study protocol conforms to the Standard Protocol Items: Recommendations for interventional Trials (SPIRIT) checklist.
Ethics approval and consent to participate
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by the ethics committee at the University of Regensburg. The committee consented to study execution on 19 February 2020, and the reference number is 20-1711-101.
Before any data was gathered, written informed consent was obtained from all participants and their legal guardians. Voluntary participation is mandatory and withdrawal from the study is possible at any time. Saliva samples given to the external laboratory for analysis are submitted, numbered and without any possibility of allocation.
This study is registered with the German Clinical Trials Register; the date of registration was 8 April 2020 and the registration number is DRKS00020907.
Eligibility criteria
Eligibility criteria are screened twice before study participation: initially in the first contact via telephone, and a second time immediately before study participation at the first study appointment (eligibility screen, see Fig. 1).
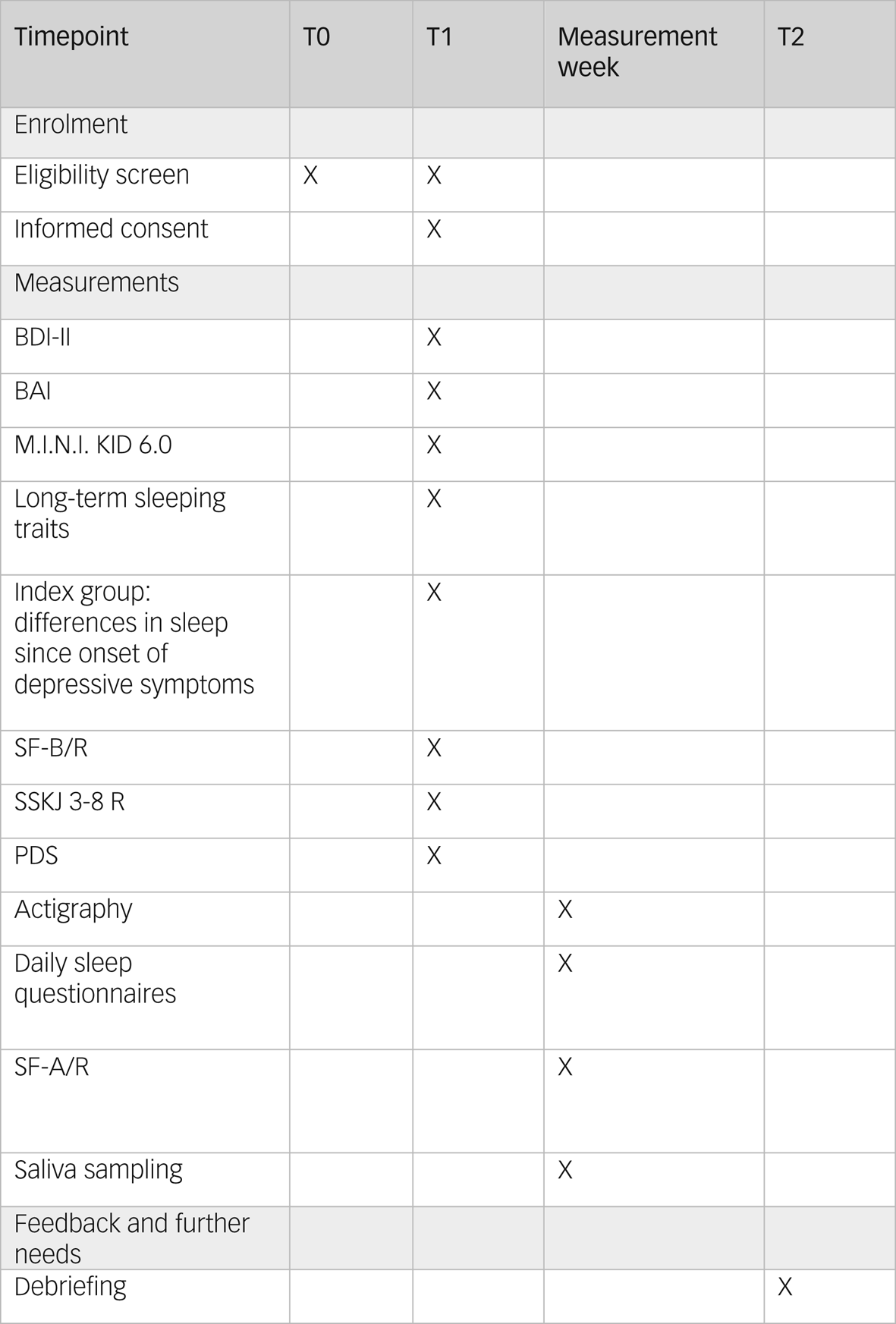
Fig. 1 Study schedule. BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory-II; M.I.N.I KID 6.0, Mini-International Neuropsychiatric Interview for Children and Adolescents; PDS, Pubertal Development Scale; SF-A/R, Sleep Questionnaire A – revised version; SF-B/R, Sleep Questionnaire B – revised version; SSKJ 3-8 R, Questionnaire for the Measurement Stress and Coping in Children and Adolescents – revised version.
Inclusion criteria
Children and adolescents between 12 and 18 years are eligible for participation. A sufficient understanding of the German language, as well as informed consent of both participants and legal guardians, is required to participate in the study.
For the index group, potential participants are those with depressive disorders according to the diagnostic criteria of the ICD-10.33 More precisely, we include the ICD-10 codes F32.0 (mild depressive episode), F32.1 (moderate depressive episode), F32.2 (severe depressive episode without psychotic symptoms), F33.0 (major depressive disorder, recurrent, mild), F33.1 (major depressive disorder, recurrent, moderate), F33.2 (major depressive disorder, recurrent, severe without psychotic features) and F43.21 (adjustment disorder with prolonged depressive reaction) in our index group.
For the control group, we include healthy adolescents matched by gender, age and pubertal status according to the Pubertal Development Scale (PDS). This shall align age- and gender-specific differences.Reference Baglioni, Nanovska, Regen, Spiegelhalder, Feige and Nissen34
Exclusion criteria
Potential participants who suffer from psychotic or acute suicidal conditions are excluded from study participation. Pregnancy, antidepressant or glucocorticoid medication, and neurological or endocrinological illnesses that may affect brain physiology or relevant endocrinological parameters are additional exclusion criteria. Moreover, those with cannabis consumption within the 3 months before study participation are excluded.
Furthermore, most psychiatric comorbidities constitute exclusion criteria. For the index group, we include anxiety disorders, as they appear cumulatively comorbid with depressive disorders.Reference Fava, Alpert, Carmin, Wisniewski, Trivedi and Biggs35,Reference Choi, Kim and Jeon36 All other psychiatric comorbidities lead to exclusion.
For the control group, we exclude participants with any psychiatric disease, symptoms indicating a current psychiatric disease, a history of psychotherapeutic or psychiatric intervention, or a condition requiring therapeutic support. Potential participants excluded because of the latter criterion are offered therapeutic help if the indication is given.
Recruitment
In the index group, participants with a diagnosed depressive disorder are consecutively recruited from the patient cohort of the out-patient department and day patient department of the Clinic of Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy at the University of Regensburg, Germany.
To recruit healthy adolescents for the control group, we will announce the study and call for participation via mailing lists, websites and social media channels of our hospital.
In case of drop-out, recruitment will be continued using the abovementioned resources until the intended number of participants is achieved.
Informed consent
No participant is included in the study unless written informed consent of the participants and legal guardians is given. If consent is revoked at any point during or after participation, all examinations of this study will be cancelled immediately for this participant.
Measurements
Assessment of depressive symptoms, psychiatric comorbidities and stress responsiveness
Initially, all participants are screened and interviewed with the Mini-International Neuropsychiatric Interview for Children and Adolescents (M.I.N.I. KID 6.0)Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller37 for psychiatric disorders (controls) or comorbidities (patients) (T1, see Fig. 1). The M.I.N.I. KID 6.0 interview is structured into 24 diagnostic modules assessing potential symptoms of psychiatric disorders according to the DSM-IV and ICD-10.
Depressive symptoms are assessed via the Beck Depression Inventory-II,Reference Hautzinger, Keller and Kühner38 which is one of the most commonly used self-rating scales to measure depressive symptomsReference Richter, Werner, Heerlein, Kraus and Sauer39 from the age of 13 years (T1, see Fig. 1). Frequently observed symptoms are inquired for the past 2 weeks in 21 items, with four possible response options covering increasing symptom severity. As an adjustment for the younger study participants, we excluded item 21 (loss of interest in sexuality) in our assessment. Internal consistency for the German version is good (α ≥ 0.84), retest reliability is r ≥ 0.75 and discriminative validity is confirmed.Reference Kühner, Bürger, Keller and Hautzinger40 The applicability to an adolescent sample was checked and validated.Reference Dolle, Schulte-Körne, O'Leary, von Hofacker, Izat and Allgaier41,Reference Pietsch, Hoyler, Frühe, Kruse, Schulte-Körne and Allgaier42
As symptoms of anxiety and anxiety disorders occur often concurrently with depressive disorders,Reference Fava, Alpert, Carmin, Wisniewski, Trivedi and Biggs35,Reference Choi, Kim and Jeon36 we examine anxiety via the Beck Anxiety Inventory (BAI)Reference Margraf and Ehlers43 (T1, see Fig. 1). The BAI consists of 21 items focusing on somatic symptoms of anxiety experienced within the past week.Reference Beck, Epstein, Brown and Steer44 The German BAI showed reliable and valid psychometrics.Reference Geissner and Huetteroth45 For adolescent samples, the BAI showed at least acceptable psychometric properties in other language versions.Reference Geissner and Huetteroth45,Reference Osman, Hoffman, Barrios, Kopper, Breitenstein and Hahn46
Stable sleeping habits and needs will be assessed by a short self-designed self-report survey (T1, see Fig. 1). This survey assesses chronotype, the regularity of bedtimes, the duration of sleep needed to feel rested and the frequency of sleeping through school or work. Furthermore, it queries the usual bed and wake-up times for each school/working days and weekend days/holidays. The items used for this survey are based on the Sleep Habit Survey (SHS).Reference Hamann, Rusterholz, Studer, Kaess and Tarokh47 For the subscales, the original SHS showed acceptable internal consistency, with α ranging from 0.70 to 0.79.Reference Kandel and Davies48
Participants with a depressive disorder additionally answer a self-designed questionnaire examining potential differences in relevant aspects of sleep quality since the onset of depressive symptoms (T1, see Fig. 1). This questionnaire consists of eight items, in which the participants compare the need to sleep, sleep and dream quality, WASO, mood at awakening, time of awakening and personal relevance of the topic sleep before and after symptom onset, and evaluate changes on a five-point Likert scale.
Sleeping quality of the previous 2 weeks is evaluated with the Sleep Questionnaire B (SF-B/R)Reference Görtelmeyer49 (T1, see Fig. 1). The SF-B/R is a validated 31-question survey. Five sleep indices can be calculated: difficulty falling asleep, difficulty staying asleep, premature awakening, general sleep characterisation and total sleep duration. In addition, seven factor scales (sleep quality, feeling of being refreshed after sleep, mental balance before sleep, feeling mentally exhausted before sleep, psychosomatic symptoms during the sleep phase, dream recall and sleep–wake regulation) can be assessed. The SF-B/R is designed for sleep research as well as clinical practice. Satisfactory reliability values >0.70 are shown with regard to the internal consistency of the factor levels and the retest reliability of the SF-B/R (2–4 weeks). With regard to convergent validity, medium correlations are found with construct-related domains, such as measures of well-being, depression and personality factors.Reference Görtelmeyer49
Participants also complete the Stress and Coping Questionnaire for Children and Adolescents (SSKJ 3-8 R)Reference Lohaus, Eschenbeck, Kohlmann and Klein-Heßling50 (T1, see Fig. 1). The SSKJ 3-8 R is a self-report questionnaire based on the transactional model of stress and coping developed by Lazarus and Folkman, assessing several strategies to reduce negative emotions or to change conditions in problematic or negatively experienced situations.Reference Weis and Heine51 The first part comprises an assessment of vulnerability to different stressors on four-level visual analogue scales. The second part includes five- and three-point Likert scales evaluating the usage frequency of five different coping strategies.Reference Weis and Heine51 The German version of the SSKJ 3-8 R showed sufficient reliability and validity has been confirmed.Reference Schmidt and Laessle52,Reference Eschenbeck, Kohlmann, Lohaus and Klein-Heßling53
Pubertal development is assessed with the use of the German adaptionReference Watzlawik54 of the Pubertal Development Scale (PDS)Reference Petersen, Crockett, Richards and Boxer55 as a self-report questionnaire (T1, see Fig. 1). Female participants asses the current status of breast development, pubic hair growth, growth in height and skin, and estimated level of development compared with peers. Male participants are asked to evaluate the start and level of beard growth, existence of puberty vocal changes, pubic hair growth, growth in height, and skin changes, and to compare their level of development with their peers.Reference Watzlawik54,Reference Petersen, Crockett, Richards and Boxer55 Measures of criterion validity as well as internal consistency have achieved acceptable values.Reference Watzlawik54
Accelerometry
For sleep measuring, we use the ActTrust 2 wrist actimeter (Condor Instruments) worn on the non-dominant wrist for seven consecutive nights, starting on Sunday evening (measurement week, see Fig. 2). Wearing and handling of this accelerometer is practiced by the adolescents and their legal guardians during the first study appointment. During the measurement week, participants put on the accelerometer when they go to bed in the evening. By pushing the event button, they record the time they intend to sleep and mark unexpected events or delays (for example, getting up again to go to the bathroom). While sleeping, data is recorded in 1-min intervals. The selected interval is the default of the manufacturer Condor Instruments, and is obligatory for the post-processing of sleep data. In the morning, wake-up time is recorded by pushing the event button again. Having marked the wake-up time, participants take the accelerometer off.

Fig. 2 Study diagram. BAI, Beck Anxiety Inventory; BDI-II, Beck Depression Inventory-II; CAR, cortisol awakening response; M.I.N.I KID 6.0, Mini-International Neuropsychiatric Interview for Children and Adolescents; PDS, Pubertal Development Scale; SF-A/R, Sleep Questionnaire A – revised version; SF-B/R, Sleep Questionnaire B – revised version; SSKJ 3-8 R, Questionnaire for the Measurement Stress and Coping in Children and Adolescents – revised version.
After participants return the accelerometer at the second study appointment, we check the recordings with the ActStudio software (Condor Instruments, São Paulo, Brazil, PC version 1.0.10, for Windows; see https://www.condorinst.com.br/en/actstudio-software/). In accordance with the standard procedure in sleep accelerometry, we manually identify the main sleep period.Reference Ruiz, Beijamini, Beale, Gonçalves, Vartanian and Taporoski56 Bed time, get up time, time in bed, total sleep time, onset latency, sleep efficiency, WASO, awakenings and the intensity of blue light are provided by the software. Additionally, activity data during sleep is derived from the data, as increased motor activity is associated with impaired sleep quality.Reference Meltzer, Short, Booster, Gradisar, Marco and Wolfson9
In the validation study, the ActTrust accelerometer achieved satisfactory results with a sensitivity of 95.69%, a specificity of 30.59%, an accuracy of 80.24%, a predictive value for sleep of 81.52% and a predictive value for wakefulness of 68.93%.Reference Rodrigues and Eckeli57 Regarding the correlation analysis between accelerometry and polysomnography, r = 0.491 (P < 0.001) was observed for total sleep time, r = 0.499 (P < 0.001) for wake after sleep onset, r = 0.345 (P < 0.001) for sleep efficiency and no statistical significance was observed for sleep onset latency.Reference Rodrigues and Eckeli57
Daily sleep questionnaires
To examine subjective sleep quality, participants complete a short questionnaire including 11 items, each morning after accelerometry measurements (measurement week, see Fig. 2). Participants evaluate the satisfaction with the past night's sleep, current mood and feelings of recreation, difficulties with sleeping in nocturnal awakening and stress level of the past day, on a six-point Likert scale. Information on the presence of nightmares, the consumed quantity of caffeinated beverages and the consumed quantity of alcoholic beverages will be gathered. Additionally, the questionnaire assesses sleeping and wake-up time to double check the ActTrust recordings.
On the last morning of measurements, participants answer the daily questionnaire as well as the Sleep Questionnaire A (SF-A/R)Reference Görtelmeyer49 (measurement week, see Fig. 2). The SF-A/R includes 25 questions assessing several qualitative and quantitative aspects of the past night's sleep. Similarly to the SF-B/R, sleep indices and factor scales can be calculated. We use data from both questionnaires to compare measurements of the self-designed daily sleep questionnaire with a standardised sleep survey.
Saliva sampling
To analyse the CAR and the course of alpha-amylase after awakening, saliva samples are collected. We instruct participants to collect saliva samples via Salivette Cortisol (Sarstedt, Nümbrecht, Germany; item number 51.1534.500) devices on three consecutive weekdays (Tuesday to Thursday) at three time points (measurement week, see Fig. 2). The first sampling time is immediately after awakening (base level of CAR), the second sample is collected 30 min after awakening (peak in CAR) and the last sampling time is 45 min after awakening (decrease in CAR). The chosen time intervals conform to the recommended sampling points of the expert consensus guidelines for CAR.Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner and Wüst32
At predetermined times, participants open a container equipped with a medication event monitoring system cap that records the opening time. The Salivettes are equipped with the fitting label for sampling days and time. Participants are instructed in oral and written form not to eat, drink or brush their teeth for 10 mins before collecting saliva samples.
The Salivettes are coded with weekdays and corresponding colours, and are kept separate for each weekday, to maximise clarity and simplify saliva sampling for participants. Sampling times are recorded and participants are informed about the monitoring before the implementation of measurements.
After sample collection, participants store the samples in their fridge or freezer, and return the samples on the second study appointment (T2, see Fig. 2). Then, the saliva samples are stored transitionally in the institute's freezer at −20°C. After completion of the study, the saliva samples are sent with a pseudonymised labelling to the Laboratory of the Department of Biopsychology, Technical University of Dresden, Germany, for analysis.
Physiological consistency of measurement
As all participants start measurements on a Sunday evening, the weekdays of accelerometry and saliva sampling stay constant over all participants. Measurement timing of female participants is aligned with the female menstrual cycle. Start of the most recent menstruation is assessed at the first study appointment, and all measurements are scheduled for the female participants’ luteal phase, as previous studies found evidence that CAR between women and men might be most similar during the luteal phase.Reference LeRoux, Wright, Perrot and Rusak58,Reference Benz, Meier, Mankin, Unternaehrer and Pruessner59 Moreover, use of oral contraceptives is assessed, as this may slightly blunt the CAR.Reference Bouma, Riese, Ormel, Verhulst and Oldehinkel60
Outcomes
Primary outcome
The primary outcome of our study is the difference between the index group and control group on all measures of subjective (daily self-assessment questionnaires) and objective (ActTrust measurements, sleep onset latency, sleep efficiency, WASO and awakenings) sleep quality, the pattern of the CAR and the course of alpha-amylase after awakening.
Secondary outcome
The secondary outcome of our study is the examination of the severity of depressive symptoms, further psychopathologies, and stress responsiveness at the first study appointment and its relation to sleep quality.
A priori power calculation
A priori sample size was calculated with the program G*Power version 3.1.9.4,Reference Faul, Erdfelder, Buchner and Lang61 based on a previous study by Schmidt et alReference Schmidt, Laessle and Hellhammer14 with a medium effect size of f = 0.30, an α error probability of 0.05 and two groups. To investigate group differences in the CAR through a repeated measures analysis of covariance (ANCOVA), a total of 28 participants would be required to achieve a power of 80%. In accordance with Selvi et al, we assume a medium effect size of d = 0.40 for correlational analyses of sleep quality and depressive symptoms.Reference Selvi, Aydin, Boysan, Atli, Agargun and Besiroglu62 A sample size of N = 44 was deemed necessary for statistical power of 80%. Finally, to enable the calculation of a direction-dependence analysis in case of non-normally distributed and sufficiently skewed data,Reference Wiedermann and von Eye63 we will include a sample of N = 60 participants: 30 participants with a depressive disorder and 30 healthy participants as parallel controls.
Expense allowance
Adolescents participating in our study receive a gift voucher of €50 as an expense allowance for participation.
Adverse event reporting and harms
We expect no adverse events caused by participating in our study. Medically indicated treatments will not be postponed or changed because of participation. Any serious aggravation of depressive or any other symptoms will result in drop-out and therapeutic assistance if needed. Participants and legal guardians can end study participation at any time without giving reasons, if they no longer wish to participate.
Statistical analysis
Data will be collected in accordance with the current standard of empirical research. Data will be evaluated with IBM SPSS Statistics version 25.0 for Windows (released 2017; IBM Corp, Armonk, New York). First, depending on the distribution of control variables, differences between the adolescents with depression and the control group will be computed via t-tests or Mann–Whitney U-tests. As cortisol and alpha-amylase levels are commonly skewed, a square-root transformation will be applied to achieve a normal distribution.Reference Kobayashi and Miyazaki64 Cortisol and alpha-amylase measurements will be averaged over three days and entered into the repeated measures ANCOVA as the dependent variable, with group as the independent variable.
Cortisol and alpha-amylase increase will be computed by area under the curve analyses.Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer65 For an additional measure of increase, delta scores will be computed between baseline and the highest value.Reference Kudielka, Buske-Kirschbaum, Hellhammer and Kirschbaum66,Reference Kudielka, Schommer, Hellhammer and Kirschbaum67 To determine group differences in cortisol and alpha-amylase increase, ANCOVAs will be calculated with group as an independent variable.
To examine the relationship between subjective and objective sleep quality, depressive symptoms and cortisol/alpha-amylase increase, bivariate correlations with a false discovery rate correction will be computed.Reference Hochberg and Benjamini68 Effect sizes will be reported with Cohen's d, with 0.2 being considered a small, 0.5 a medium and 0.8 a large effect.
Finally, in case of non-normality distributed and sufficiently skewed outcome variables, the direction of the relationship between sleep quality and depressive symptoms will be examined with direction dependence analysis.Reference Wiedermann and von Eye63 Direction dependence analysis enables to statistically test the directional relation between pairs of variables, including observational variables, as well as adjusting for covariates that may contribute to the causal process.Reference Wiedermann and Li69
Discussion
Knowledge gains
Although sleep disturbance and cortisol release has been the object of broad research in the context of depressive disorders in past years,Reference Lopez-Duran, Kovacs and George70,Reference Stroud, Vrshek-Shallhorn, Norkett and Doane16 to the best of our knowledge, no study has examined the combination of subjective sleep quality, accelerometry measuring objective sleep quality, and neurohormonal correlates of HPA axis and autonomous nervous system activity in adolescents with depression before. With the results of our study, we aim to better understand how the strength and course of cortisol and alpha-amylase awakening responses, and subjective as well as objective sleep quality, are related in adolescents with depressive disorders. This present study design allows a wide-ranging approach to sleep impairment and the status of different physiological stress responses. Considering the importance of sleep in the process of growth and maturation, this knowledge is urgently needed to enhance the perspective for adolescent patients with depression, and to integrate sleep quality more profoundly into the treatment process.Reference Baglioni, Nanovska, Regen, Spiegelhalder, Feige and Nissen34,Reference Rao7,Reference Goldstein, Bridge and Brent6
Relevance of the study
The major value of this study is its contributions toward clinical practice and patients with depressive disorders. In the recruitment process for patients with depression, we receive valuable feedback from both patients and legal guardians about the relevance and level of suffering sleep impairment causes for adolescents with depression. In clinical practice, the urgent demand for targeted assessment strategies and adequate treating to reduce patients’ suffering becomes apparent. Moreover, practising therapists and physicians are frequently confronted with the topic of sleep impairment, but current research on the characteristics of these sleep concerns, the actual amount of objective disruption and subjectively experienced impairment, and especially its effects on different physiological stress systems, does not meet patients’ needs. Previous studies and meta-analyses have repeatedly confirmed these deficits.Reference Goldstein, Bridge and Brent6,Reference Rao7,Reference Baglioni, Nanovska, Regen, Spiegelhalder, Feige and Nissen34,Reference Lofthouse, Gilchrist and Splaingard71 The results of the current study are of great value to better our understanding of the origin and overall impact of impaired sleep quality. Moreover, the findings can be a useful reference point for the development of sleep assessments and sleep diaries, which particularly meet the needs of adolescent patients with depressive disorders.
In the pathophysiology regarding trigger and recurrence of depressive disorders, CAR seems to constitute a promising and highly relevant biomarker linked to coping and daily hassles.Reference Nater, La Marca, Florin, Moses, Langhans and Koller22 An increased CAR in healthy adolescents might be a useful mechanism to adjust to temporarily increased daily demands and to reduce the individual stress level.Reference Nater, La Marca, Florin, Moses, Langhans and Koller22 A potential dysregulation of this pathomechanism could be highly relevant in the context of stress and occurrence of depressive symptoms.Reference Dedovic and Ngiam31 Because of these previous findings, we added the SSKJ 3-8 R questionnaire to our assessment, which examines vulnerability to daily hassles and the use of various coping mechanisms.Reference Weis and Heine51
In the context of stress, salivary alpha-amylase has been shown to have a sensitive response to acute stress situations,Reference Nater, La Marca, Florin, Moses, Langhans and Koller22 with a potentially higher specificity for depressive disorders.Reference Bauduin, van Noorden, van der Werff, de Leeuw, van Hemert and van der Wee23
Considering that the onset of depressive symptoms is often linked to a major or minor stressful event,Reference Dedovic and Ngiam31 the examination of pathophysiological aspects of these stress-related biomarkers in adolescents with depressive disorders is essential. By examining the neurohormonal correlates, we aim to contribute to the examination of the relationship between the hormonal system and sleep quality of adolescents with depressive disorders.
All participants in this study will benefit from a detailed and technically supported analysis of sleep quality, which exceeds the diagnostic standard in depressive disorders by far.
Strengths and limitations
One particular strength of our study is the domestic sleep setting. with its simple and non-invasive accelerometry measurement, which preserves the familiar sleeping habits and allows us to assess sleeping measurements with as little distortion as possible.Reference Meltzer, Short, Booster, Gradisar, Marco and Wolfson9,Reference Lofthouse, Gilchrist and Splaingard71 By choosing out-patients or day patients for the index group, we strive for an undisturbed and natural sleeping condition,Reference McCall, Reboussin and Cohen10 to achieve generalisability of the study findings. Additionally, the combination of an accelerometry measurement and CAR as well as alpha-amylase measurements after awakening provides the benefit of an objective validation of awakening, which is a useful verification for the saliva sampling times.Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner and Wüst32,Reference Bauduin, van Noorden, van der Werff, de Leeuw, van Hemert and van der Wee23 Another strength is the broadness of objective and subjective measurement methods combined with the assessment of both HPA axis and autonomous nervous system activation,Reference Rao7 which is, to our knowledge, a rare approach in research focusing on adolescent patients with depression.Reference Jezova, Trebaticka, Buzgoova, Durackova and Hlavacova24 With the heterogeneous findings of previous sleep research in adolescent patients with depressionReference Rao7,Reference Gupta, Sagar and Mehta11 and the unexplored potential of alpha-amylase as a useful addition to cortisol-based stress research,Reference Nater, La Marca, Florin, Moses, Langhans and Koller22 the findings of this study can be of great value to the consideration of depression and physiological stress conditions and its consequences, and may stimulate further research.
A considerable limitation of this study is the exclusion of adolescents with depression who are on antidepressant medication in favour of saliva sampling and including only out-patients and day patients. These eligibility criteria may shift the examined cohort to less severe courses of depressive diseases and exclude long-term and severely ill patients who often take antidepressant medicine or undergo in-patient treatment. Nevertheless, with a prompt study advertisement and a contemporary study appointment, we are able to include a wide range of depression severity before medication intake or in-patient admission is decided. An additional limitation is that external interfering factors disturbing participants’ sleep cannot be controlled in the domestic surrounding during the measurement week. However, these external influences are unavoidable when data is collected in the natural surroundings and under everyday conditions. Subjective sleep impairment in adolescents with depression is mostly experienced within their home environment, thus examining sleep quality, including all external influences, is crucial for a realistic and reliable assessment, and leads to a high ecological validity. Finally, measurements of sleep and salivary biomarkers are limited to 1 week or 3 days, respectively, which implies a certain variability in the course of disease and external influences. Nonetheless, the 3-day saliva sampling and the 1-week sleep measurements constitute a detailed and wide-ranging assessment compared with previous assessments,Reference van Lenten and Doane30 and can therefore be considered reliable.
Despite the aforementioned limitations, this study adds crucial and multifactorial value to the pre-existing research in sleep and stress research in adolescents with depressive disorders. In addition, the results of our study could make an important contribution in the establishment of an ambulatory assessment of sleep quality and continuous observation of sleep impairment in the course of disease in adolescents with depressive disorders.
Trial status
Recruitment has started on 1 February 2021, and the study is intended to be completed by February 2022.
Data availability
The data-sets used and/or analysed during the current study are available from the corresponding author, S.K., on reasonable request.
Acknowledgements
Many thanks go to neuroCare (Illmenau) for their support with the ActTrust accelerometers. We also thank Prof. Dr Clemens Kirschbaum (Department of Biopsychology, Technical University of Dresden, Germany) and the Laboratory of the Department of Biopsychology, Technical University of Dresden, for their support and laboratory examination.
Author contributions
R.K. participated in designing and coordinating the study, and wrote the first draft of the manuscript. S.K. participated in study design and coordination, and helped with writing the manuscript. R.B., D.S. and A.E. participated in study design and coordination, and revised the manuscript critically. I.J. was responsible for the statistical analyses and the sample size calculation, and revised the article critically. The final manuscript was revised and approved by all authors.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.






eLetters
No eLetters have been published for this article.