Epilepsy is common in adults with intellectual disabilities, with an average estimated point prevalence of 25%, compared with <1% in the general population who do not have intellectual disabilities.Reference Deb1 The rate increases with the severity of disability:Reference Deb and Joyce2 around 7–15% in mild to moderate intellectual disability, 67% in severe intellectual disability and 82% in profound intellectual disability. The rate increases if the intellectual disability is associated with other neurological disorders, such as cerebral palsy.Reference Deb1
Special issues relating to epilepsy in adults with intellectual disabilities
Certain genetic syndromes that lead to intellectual disabilities, such as Angelman, Sturge–Weber, fragile-X/ataxia, Rett, Lesch–Nyhan, Rubinstein–Taybi, Lowe and Down syndromes and tuberous sclerosis, are commonly associated with epilepsy.Reference Clarke, Deb, Gelder, Andreasen, López-Ibor and Geddes3 A high proportion of people with autism spectrum disorder (22%) also have epilepsy.Reference Deb1 Similarly, certain epilepsy syndromes, such as West syndrome (in infants), Lennox–Gastaut syndrome, Landau–Kleffner syndrome and Dravet syndrome, are more commonly associated with intellectual disabilities.Reference Berney, Deb, Shorvon, Guerrini, Cook and Lahtoo4 Compared with the general population of adults who do not have intellectual disabilities, epilepsy among adults with intellectual disabilities is not only more prevalent, but it also often manifests as multiple seizure types, starts at an early age, is of longer duration and is resistant to anti-epileptic treatment (in over 30% in the general population, compared with over 70% in intellectual disabilities).Reference Shankar, Watkins, Alexander, Devapriam, Dolman and Hari5 Diagnosing epilepsy and seizure type can be difficult in this population, and both false-positive (stereotypy, cardiac syncope, non-epileptic attack disorder may all mimic epileptic seizure) and false-negative (difficulty diagnosing absence, focal seizures) diagnoses are possible.Reference Deb, Jacobson and Maulick6 Also, these people are more prone to die from sudden unexpected death in epilepsy (SUDEP).Reference Shankar, Eyeoyibo, Scheepers, Dolman, Watkins and Attavar7, Reference Kerr, Mensah, Besag, De Toffol, Ettinger and Kanemoto8
Relationship between epilepsy and challenging behaviour in adults with intellectual disabilities
The relationship between epilepsy and challenging behaviour in adults with intellectual disabilities is complex.Reference Deb, Bouras and Holt9 Challenging behaviour has been defined as ‘socially unacceptable behaviour that causes distress, harm or disadvantage to the persons themselves or to other people, and usually requires some intervention’.Reference Deb, Kwok, Bertelli, Salvador-Carulla, Bradley and Torr10 Challenging behaviour is prevalent among adults with intellectual disabilities, affecting up to around 62%.Reference Smith, Branford, Collacott, Cooper and McGrother11–Reference Hemmings, Deb, Chaplin, Hardy and Mukherjee14 More severe forms of challenging behaviour are manifested by a lower proportion (18.7–30%).Reference Lundqvist13, Reference Deb, Deb, Faruqui, Bodani and Agrawal15 The types of challenging behaviour include aggression, destruction of property, disruptive behaviour, self-injurious behaviour, stereotypy, and sexually inappropriate and harmful behaviours.Reference Hemmings, Deb, Chaplin, Hardy and Mukherjee14, Reference Deb, Bethea, Havercamp, Rifkin, Underwood, Fletcher, Barnhill and Cooper16 Aggression is reported in 10–20% of adults with intellectual disabilities.Reference Deb, Bethea, Havercamp, Rifkin, Underwood, Fletcher, Barnhill and Cooper16, Reference Sjgafoos, Elkins, Kerr and Attwood17 The aetiology of challenging behaviour is multifactorial, including medical, psychiatric, psychological, social and environmental factors. Therefore, a multiprofessional approach is required to formulate a person-centred treatment plan for this difficult-to-manage problem.Reference Deb, Bethea, Havercamp, Rifkin, Underwood, Fletcher, Barnhill and Cooper16 Demographic factors such as age, gender, severity of intellectual disability, associated comorbidities (e.g. other neurodevelopmental disorders such as autism spectrum disorder, attention-deficit hyperactivity disorder), medical conditions and psychosocial factors can all affect challenging behaviour in adults with intellectual disabilities.Reference Hemmings, Deb, Chaplin, Hardy and Mukherjee14, Reference Deb, Unwin, Rojahn, Cooper, Bertelli, Deb, Munir, Hassiotis and Salvador-Carulla18 Epilepsy is one such factor that may influence a person's behaviour.
Previous systematic reviews on the subject
Three systematic reviews looked at the association between challenging behaviour and epilepsy in people with intellectual disabilities: none of these found any association.Reference Blickwedel, Ali and Hassiotis19–Reference van Ool, Snoeijen-Schouwenaars, Schelhaas, Tan, Aldenkamp and Hendriksen21 We decided to carry out an updated systematic review, as important publications either have appeared since the last reviews or were not included in those reviews. Another reason for carrying out this review is to conduct meta-analyses that were not done in any of the previous reviews.
Method
The aim of the current systematic review and meta-analysis is to identify the rates and types of challenging behaviour in adults with intellectual disabilities who have epilepsy (‘the epilepsy group’) and compare them with rates and types in adults with intellectual disabilities who do not have epilepsy (‘the non-epilepsy group’) to determine whether epilepsy is a risk factor for developing challenging behaviour in this population.
We aimed to include studies that compared the overall rate of challenging behaviour as well as different types of challenging behaviour in adults with intellectual disabilities with and without epilepsy within the same cohort. We did not aim to include any study that involved only adults with intellectual disabilities who did not have epilepsy. We included studies that involved participants without epilepsy only where they were part of the control group and participants with epilepsy were also involved in the same study.
We also aimed to include studies that involved adults with intellectual disabilities and epilepsy but no one without epilepsy. These studies are included to assess the rate of different types of challenging behaviour according to different seizure variables.
Search strategy
We decided on our final search strategy after an initial scoping literature search. We followed PROSPERO guidelines22 and the PRISMA-P checklist to develop our protocol and search strategy.Reference Moher, Shamseer, Clarke, Ghersi, Liberati and Petticrew23 Five electronic databases – Embase, PubMed/MEDLINE, PsycInfo, DARE and ASSIA (ProQuest) – were searched for relevant journal articles. Each database was searched between 1 January 1985 and 31 May 2020. In addition, we also cross-referenced pertinent reviews and articles. We hand-searched for relevant articles in the past 20 years’ issues, from January 1990 to May 2020, in the following intellectual disabilities journals: (a) Journal of Intellectual Disability Research, (b) Journal of Applied Research in Intellectual Disabilities (JARID) and (c) Research in Developmental Disabilities; and the following epilepsy journals: (a) Seizure, (b) Epilepsy & Behavior and (c) Epilepsia.
Only quantitative studies in English were searched. We excluded non-human studies, studies involving children and conference abstracts.
Search terms
The list of search terms used can be found in the supplementary material available at https://doi.org/10.1192/bjo.2020.96. Search terms were adapted from previous systematic reviews that were carried out to develop a national and an international guide for the use of psychotropic medications in the management of challenging behaviour in adults with intellectual disabilities.Reference Deb, Kwok, Bertelli, Salvador-Carulla, Bradley and Torr10, Reference Unwin and Deb24
Criteria for selecting studies
We devised a list of eligibility criteria based on PROSPERO22 and Cochrane review guidelinesReference Lefebvre, Glanville, Briscoe, Littlewood, Marshall, Metzendorf, Higgins, Thomas, Chandler, Cumpston, Li and Page25 and adapted from similar systematic reviews on psychotropic medicationsReference Deb, Farmah, Arshad, Deb, Roy and Unwin26–Reference Unwin and Deb28 (supplementary Appendix 1).
Types of study
There was no restriction on the type of study design included in this review. We included both randomised and non-randomised studies; controlled and non-controlled observational or cross-sectional studies; and controlled studies with both matched and non-matched control groups.
Types of participant
All participants had intellectual disabilities, were aged 16 years or over and displayed various types of challenging behaviour. We have only included data on challenging behaviour in this review. Data on psychiatric illness without challenging behaviour are not presented in the current paper as they will be included in a separate systematic review article.
No ethical approval was required for this study as no individual patient-related data were collected or analysed.
Sample size
Studies with fewer than 10 participants were excluded. This arbitrary cut-off was used in accordance with our previous systematic reviews.Reference Deb, Kwok, Bertelli, Salvador-Carulla, Bradley and Torr10
Secondary outcome
The rates of different types of challenging behaviour (verbal aggression and aggression towards people or property or self, stereotypy, overactivity, temper tantrum, etc.) were compared between the epilepsy and the non-epilepsy groups. Data on subgroup comparisons according to different types of seizure, seizure frequency and different pharmacological regimes (e.g. polypharmacy versus monopharmacy of anti-epileptic medications; treatment with carbamazepine versus valproate) were collected to identify the role of different epilepsy-related factors in the development of challenging behaviour.
Selection process
After the definitive search was completed, titles were searched for key terms. Zotero reference management software29 was used to manage and record references from each database. Duplicates, non-human studies and studies involving children were identified by Zotero and removed manually (by B.A.B.). The remaining abstracts were screened independently using the pre-piloted eligibility criteria (by B.A.B. and B.L.). The two authors were masked to each other's scores. Bibliographies of potential studies were screened to identify articles that required acquisition of the full text. Discrepancies identified were reviewed and discussed between B.A.B. and B.L. to resolve any differences. The full texts were then reviewed and independently assessed for eligibility by B.A.B. and B.L. using the same eligibility checklist that was used for screening the abstracts. The selection process is shown in Fig. 1. It was not necessary for the third review author (S.D.) to arbitrate.

Fig. 1 The flowchart of the paper selection process.
Data extraction
Data from studies meeting eligibility criteria were extracted independently by B.A.B. and B.L. using a standardised data extraction template adapted from Cochrane review guidelines (supplementary Appendix 2).Reference Li, Higgins, Deeks, Higgins, Thomas, Chandler, Cumpston, Li and Page30 S.D. checked this information for accuracy. Results are reported using a narrative synthesis of information from included studies.
Meta-analysis
The meta-analysis was carried out only on controlled studies. An odds ratio was calculated for the studies that presented the proportion of participants in each group displaying challenging behaviour. A standardised mean difference was calculated for those studies that presented means and standard deviations for scores based on a behaviour rating scale in the two groups.Reference Deeks, Higgins, Altman, Higgins, Thomas, Chandler, Cumpston, Li and Page31 To pool data, we log-transformed all data to standardised mean differences.Reference Deeks, Higgins, Altman, Higgins, Thomas, Chandler, Cumpston, Li and Page31 RevMan 5.332 software for Windows 10 was used for random-effects meta-analysis. Heterogeneity was tested using the χ2-test and I 2-statistic. Where there was substantial heterogeneity (I 2 > 60%), a further sensitivity analysis was carried out. A final meta-analysis was carried out after removing data from the studies that produced a high heterogeneity and strong bias, to bring heterogeneity to an acceptable level (I 2 < 30).
Risk-of-bias assessment and confidence in cumulative estimates
The risk of bias for the 19 controlled studies identified was assessed independently by B.A.B. and B.L. using the Cochrane risk-of-bias toolReference Sterne, Savović, Page, Elbers, Blencowe and Boutron33 and the quality of all 32 eligible studies was assessed during the data collection process using the SIGN 50 checklist.34
Publication bias was assessed using a funnel plot, and the studies included were assessed for consistency and precision. The quality of the overall systematic review was assessed using AMSTAR 2 criteria (see Appendix 3; supplementary material).Reference Shea, Reeves, Wells, Thuku, Hamel and Moran35
Results
Included studies
Of the 868 articles screened, 34 papers (from 32 studies) met the eligibility criteria and were included in the systematic review (Fig. 1). A list of excluded articles with reason for exclusion is presented in supplementary Appendix 4. Two studies each published two papers on the same sample but on different outcome measures. Of the 34 papers, 19 included participants with and without epilepsy, as their authors compared the rates of challenging behaviour in these two groups to assess an association between challenging behaviour and epilepsy (Table 1). Of these, nine studiesReference Deb, Cowie and Richens36–Reference Smith and Matson44 had equal numbers of participants in both groups and the groups were matched on various demographic variables. The rest (n = 10)Reference Deb, Thomas and Bright12, Reference Prasher45–Reference Blickwedel, Vickerstaff, Walker and Hassiotis53 were prevalence studies of challenging behaviour in adults with intellectual disabilities that included a number of participants with epilepsy (around 22% of the cohort). Different variables, such as age, gender and presence/absence of epilepsy, were used to assess whether they are risk factors for developing challenging behaviour. The rates of challenging behaviour were compared on the basis of the presence or absence of epilepsy, but the epilepsy groups were not matched with the non-epilepsy groups. The rest of the studies included participants with epilepsy only, and in these the rates of challenging behaviour were compared between various types of epilepsy (e.g. frequent versus infrequent; generalised versus focal seizures). Table 2 compares the rates of different types of challenging behaviour (e.g. aggression, self-injurious behaviour) between the epilepsy and the non-epilepsy groups. Table 3 presents data on participants with epilepsy only and compares rates of challenging behaviour according to various epilepsy variables. Most studies were carried out in the UK (n = 24), some in the USA (n = 4) and one each in Ireland, Sweden, The Netherlands and Spain. In total, data on 14 168 adults with intellectual disabilities are presented (4781 with epilepsy and 9387 without epilepsy).
Table 1 Rates of challenging behaviour in adults with intellectual disabilities with and without epilepsy

ABC-C, Aberrant Behaviour Checklist-Community version; ABS-II, Adaptive Behaviour Scale Part II; ANOVA, analysis of variance; ANCOVA, analysis of covariance; ASD-CA, Autism Spectrum Disorders-Comorbidity-Adult version battery; DAS, Disability Assessment Schedule; DASH-II, Diagnostic Assessment for the Severely Handicapped-Part 2; MANOVA, multivariate analysis of variance; MANCOVA, multivariate analysis of covariance; PAA, Profile of Abilities and Adjustment schedule; PBS, Psychosocial Behaviour Scale; SIB, self-injurious behaviour.
Table 2 Types of challenging behaviour in adults with intellectual disabilities with and without epilepsy
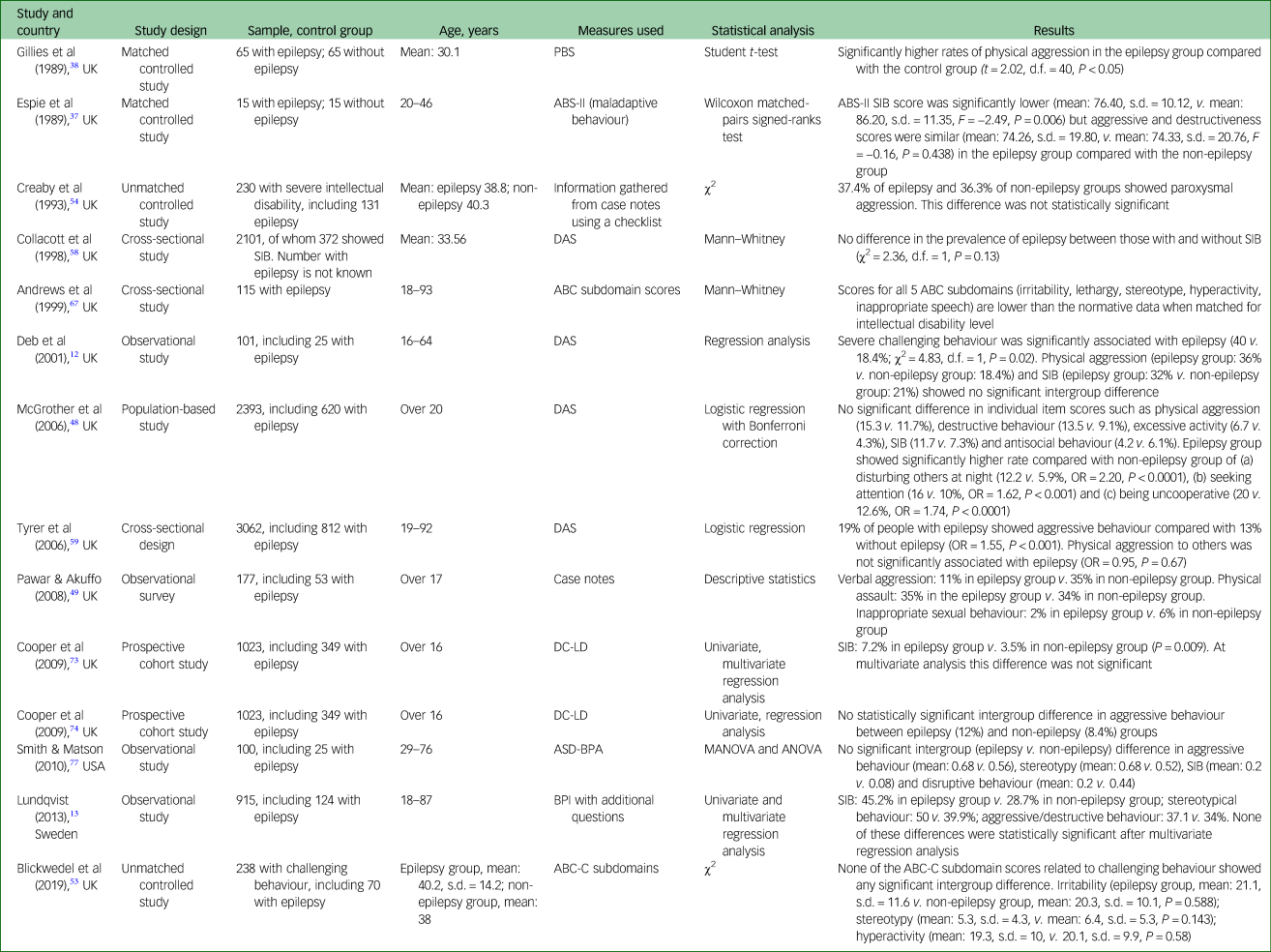
ABC-C, Aberrant Behaviour Checklist-Community version; ABS-II, Adaptive Behaviour Scale Part II; ANOVA, analysis of variance; ASD-BPA, Autism Spectrum Disorders-Behaviour Problem-Adult version; BPI, Behavior Problem Inventory; DAS, Disability Assessment Schedule; DC-LD, Diagnostic Classification-Learning Disability; MANOVA, multivariate analysis of variance; PBS, Psychosocial Behaviour Scale; SIB, self-injurious behaviour.
Table 3 Challenging behaviours in adults with intellectual disabilities according to different epilepsy variables
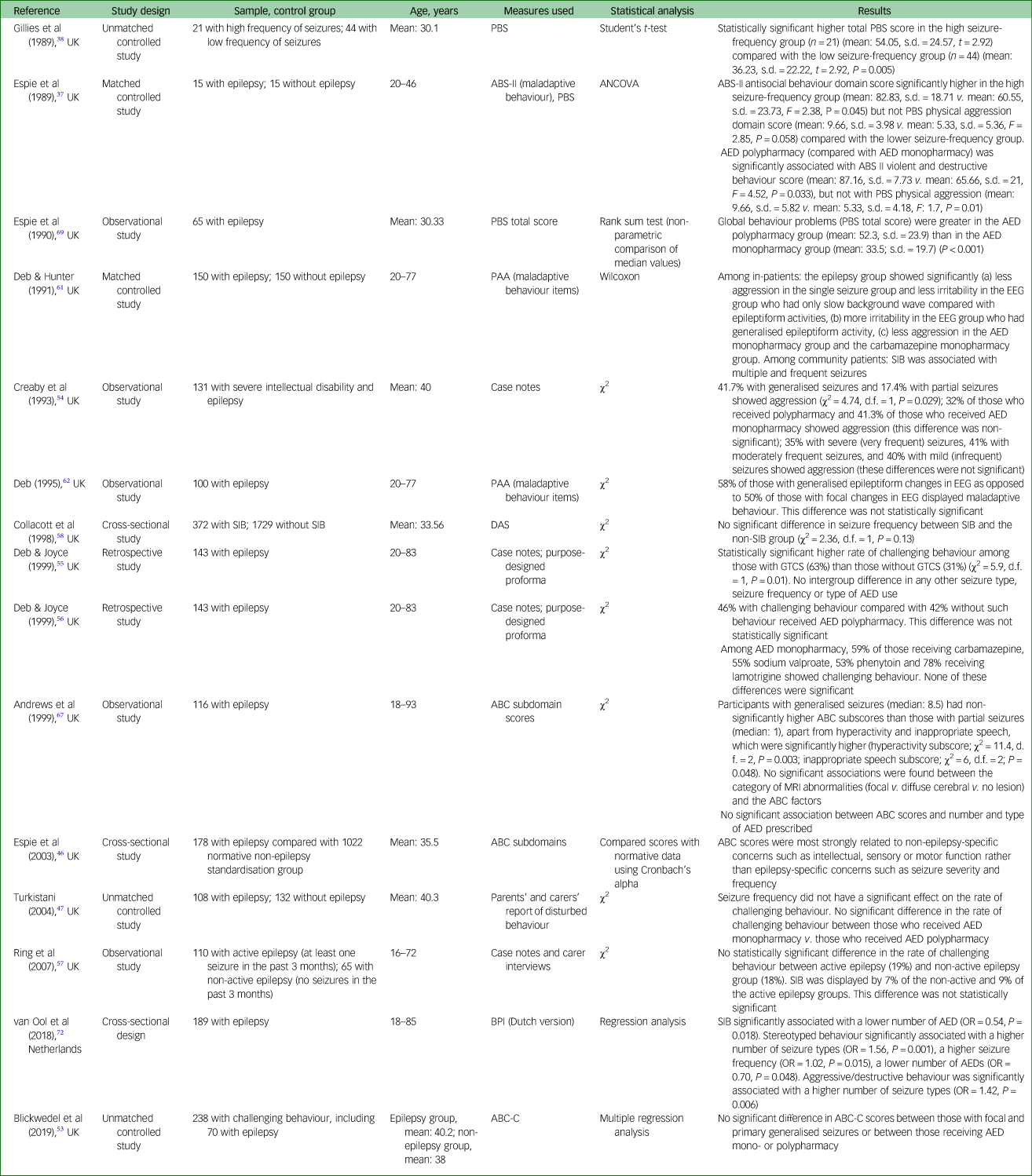
ABC-C, Aberrant Behaviour Checklist-Community version; ABS-II, Adaptive Behaviour Scale Part II; AED, anti-epileptic drugs; ANCOVA, analysis of covariance; BPI, Behaviour Problem Inventory; DAS, Disability Assessment Schedule; EEG, electroencephalogram; MRI, magnetic resonance imaging; PAA, Profile of Abilities and Adjustment schedule; PBS, Psychosocial Behaviour Scale; SIB, self-injurious behaviour; GTCS, generalised tonic–clonic seizures.
Diagnosis
Challenging behaviour is defined using a variety of methods in different studies. Five studiesReference Pawar and Akuffo49, Reference Creaby, Warner, Jamil and Jawad54–Reference Ring, Zia, Lindeman and Himlok57 collected data retrospectively from participants’ case notes and did not use any validated measures. One studyReference Turkistani47 collected information on challenging behaviour from parents’ and carers’ reports and one studyReference O'Dwyer, McCallion, Burke, Carroll, O'Dwyer and McCarron51 used its own questionnaire, for which no validation data are provided. Among the studies that used validated questionnaires, fiveReference Deb, Thomas and Bright12, Reference Deb, Cowie and Richens36, Reference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48, Reference Collacott, Cooper, Branford and McGrother58, Reference Tyrer, McGrother, Thorp, Donaldson, Bhaumik and Watson59 used challenging behaviour items from the Disability Assessment Schedule (DAS)Reference Holmes, Shah and Wing60 and another threeReference Deb40, Reference Deb and Hunter61, Reference Deb62 used DAS challenging behaviour items from the Profile of Abilities and Adjustment schedule (PAA).Reference Deb and Hunter61, Reference Wing63 Three studiesReference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Collacott39, Reference Prasher45 used the Adaptive Behaviour Scale Part II (ABS-II),Reference Nihira, Foster, Shellhaas and Leland64 which scores maladaptive behaviours. Two studiesReference Matson, Bamburg, Mayville and Khan41, Reference Fitzgerald, Matson and Barker50 used the Diagnostic Assessment for the Severely Handicapped Part 2 (DASH-II).Reference Matson, Gardner, Coe and Sovner65 Two studiesReference Matthews, Weston, Baxter, Felce and Kerr43, Reference Blickwedel, Vickerstaff, Walker and Hassiotis53 used the Aberrant Behaviour Checklist-Community version (ABC-C) total score,Reference Aman, Burrow and Wolford66 and another fourReference Chung and Cassidy42, Reference Espie, Watkins, Curtice, Espie, Duncan and Ryan46, Reference Folch, Cortés, Salvador-Carulla, Vicens, Irazábal and Muñoz52, Reference Andrews, Everitt and Sander67 used ABC-C subdomain scores.Reference Aman and Singh68 Three studiesReference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Gillies, Espie and Montgomery38, Reference Espie, Gillies and Montgomery69 used the Psychosocial Behaviour Scale (PBS).Reference Espie, Montgomery and Gillies70 The Behaviour Problem Inventory (BPI)Reference Rojahn, Matson, Lott, Esbensen and Smalls71 was used in two studiesReference Deb, Thomas and Bright12, Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 to rate challenging behaviour. One study each used the Diagnostic Criteria-Learning Disability (DC-LD),Reference Cooper, Smiley, Allan, Jackson, Finlayson and Mantry73–75 Autism Spectrum Disorders-Comorbidity-Adult version (ASD-CA)Reference Smith and Matson44, Reference Matson, Terlonge and González76 and Autism Spectrum Disorders-Behaviour Problems for Adults (ASD-BPA)Reference Smith and Matson77, Reference Matson and Rivet78 respectively.
Statistical methods used
Where a standardised scale was used to measure challenging behaviours, some studies compared means and standard deviations for the two groups, whereas others used an arbitrary cut-off score to compare participants in the two groups.Reference Coolican79 Various statistical methods were used, including χ2, regression analysis, univariate and multivariate regression analyses, the Wilcoxon matched-pairs signed-ranks test and Student's t-test.
Outcome (narrative synthesis)
The overall rate of challenging behaviour
Of the total 19 controlled studies, 13Reference Deb, Thomas and Bright12, Reference Deb, Cowie and Richens36, Reference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Collacott39, Reference Deb40, Reference Matthews, Weston, Baxter, Felce and Kerr43–Reference Prasher45, Reference Turkistani47, Reference Fitzgerald, Matson and Barker50–Reference Blickwedel, Vickerstaff, Walker and Hassiotis53 did not show any significant intergroup difference in the overall rate of challenging behaviour, threeReference Gillies, Espie and Montgomery38, Reference Chung and Cassidy42, Reference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48 showed a significantly higher rate of challenging behaviour in the epilepsy group and threeReference Matson, Bamburg, Mayville and Khan41, Reference Espie, Watkins, Curtice, Espie, Duncan and Ryan46, Reference Pawar and Akuffo49 showed a higher overall rate of challenging behaviour in the non-epilepsy group (Table 1). Of these three, oneReference Matson, Bamburg, Mayville and Khan41 was at a significant level and the level of significance for other twoReference Espie, Watkins, Curtice, Espie, Duncan and Ryan46, Reference Pawar and Akuffo49 is not known.
Rates of different types of challenging behaviour
Table 2 shows the rates of different types of challenging behaviour in the epilepsy and the non-epilepsy groups. For aggression, nine studiesReference Deb, Thomas and Bright12, Reference Lundqvist13, Reference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48, Reference Pawar and Akuffo49, Reference Blickwedel, Vickerstaff, Walker and Hassiotis53, Reference Creaby, Warner, Jamil and Jawad54, Reference Cooper, Smiley, Jackson, Finlayson, Allan and Mantry74, Reference Smith and Matson77 showed no significant intergroup difference. According to two studiesReference Gillies, Espie and Montgomery38, Reference Tyrer, McGrother, Thorp, Donaldson, Bhaumik and Watson59 the epilepsy group showed a statistically significant higher rate of aggression compared with the non-epilepsy group. In one studyReference Andrews, Everitt and Sander67 aggression was less common in the epilepsy group. For self-injurious behaviour, six studiesReference Deb, Thomas and Bright12, Reference Lundqvist13, Reference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48, Reference Collacott, Cooper, Branford and McGrother58, Reference Cooper, Smiley, Allan, Jackson, Finlayson and Mantry73, Reference Smith and Matson77 showed no significant intergroup difference and one studyReference Espie, Pashley, Bonham, Sourindhrin and O'donovan37 showed significantly less self-injurious behaviour in the epilepsy group compared with the non-epilepsy group. For aggression to property or destructiveness, three studiesReference Lundqvist13, Reference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48 showed no significant intergroup difference and no study showed a higher rate in the epilepsy group compared with the non-epilepsy group. One studyReference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48 showed a significantly higher rate of behaviours such as disturbing others at night, seeking attention and being uncooperative in the epilepsy group compared with the non-epilepsy group. Rates of behaviours such as stereotypy, inappropriate sexual behaviour, irritability, hyperactivity, verbal aggression, antisocial behaviours and lethargy. were reported in a very small number of studies showing equivocal findings.
Association between challenging behaviour and epilepsy-related variables
Table 3 presents data on the rate of challenging behaviour according to different epilepsy variables. For example, in three studiesReference Creaby, Warner, Jamil and Jawad54, Reference Deb and Hunter61, Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 a significantly higher rate is reported among those with generalised seizures as opposed to focal seizures but in one studyReference Andrews, Everitt and Sander67 the intergroup difference was not significant. Similarly, three studiesReference Gillies, Espie and Montgomery38, Reference Deb and Hunter61, Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 showed a higher rate of challenging behaviour among those who presented with frequent seizures as opposed to those who had less frequent seizures. However, in five studiesReference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Turkistani47, Reference Creaby, Warner, Jamil and Jawad54, Reference Deb and Joyce55, Reference Collacott, Cooper, Branford and McGrother58 no such significant intergroup difference was found. In one studyReference Deb and Hunter61 those who showed generalised epileptiform changes on electroencephalograms (EEGs) showed a significantly higher rate of challenging behaviour compared with those whose EEGs showed focal epileptiform changes.
Association between challenging behaviour and anti-epileptic medication-related variables
Three studiesReference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Deb and Hunter61, Reference Espie, Gillies and Montgomery69 showed a significantly higher rate of challenging behaviour among those who received multiple anti-epileptic medications (polypharmacy group) compared with those who received single anti-epileptic medication (monopharmacy group). However, no such significant intergroup difference was found in five studies.Reference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Turkistani47, Reference Blickwedel, Vickerstaff, Walker and Hassiotis53, Reference Creaby, Warner, Jamil and Jawad54, Reference Deb and Joyce56 Interestingly, two studiesReference Creaby, Warner, Jamil and Jawad54, Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 reported a higher rate of challenging behaviour in the monopharmacy group than in the polypharmacy group, and in one of these studies this intergroup difference was significant.Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 Only one study reported the rate of challenging behaviour in various monopharmacy groups (phenytoin, sodium valproate, carbamazepine and lamotrigine monopharmacy) but it made no intergroup comparison.Reference Deb and Joyce56
Meta-analysis
Of the 19 controlled studies, data were available for meta-analysis from 16. Pooled data from the 16 studies using a random-effects meta-analysis of standardised mean differences showed no significant intergroup difference but the heterogeneity among studies was very high (I 2 = 88%) (Fig. 2). After sensitivity analysis we removed data from studies that produced the highest level of heterogeneity and had a high risk of bias according to the Cochrane risk-of-bias tool. The final meta-analysis, using a random-effects model, of pooled data from the remaining 10 studies showed a statistically significant higher rate of overall challenging behaviour in the epilepsy group compared with the non-epilepsy group, with a very small effect size of 0.16. The heterogeneity among studies came down to an acceptable level (I 2 = 18%) (Fig. 3). To study specific types of challenging behaviour, we conducted meta-analyses of pooled data on aggression scores from nine studies (Fig. 4), on self-injurious behaviour from six (Fig. 5) and on stereotypy from three (Fig. 6). The aggression meta-analysis showed a significantly higher rate in the epilepsy group compared with the non-epilepsy group, with a very small effect size of 0.16. The heterogeneity level was low (I 2 = 27%). The self-injurious behaviour meta-analysis also showed a statistically significant higher rate in the epilepsy group compared with the non-epilepsy group, with a very small effect size of 0.28, but the heterogeneity score was high, albeit below 60% (I 2 = 54%). The stereotypy meta-analysis did not show any significant intergroup difference but showed a high heterogeneity value of over 60% (I 2 = 69%).

Fig. 2 Forest plot of total challenging behaviour score data from 16 studies.
NEP, no epilepsy; EP, epilepsy.

Fig. 3 Forest plot of total challenging behaviour score data from 10 studies after sensitivity analysis.
NEP, no epilepsy; EP, epilepsy.

Fig. 4 Forest plot of aggression score data.
NEP, no epilepsy; EP, epilepsy.

Fig. 5 Forest plot of self-injurious behaviour score data.
NEP, no epilepsy; EP, epilepsy.

Fig. 6 Forest plot of stereotypy score data.
NEP, no epilepsy; EP, epilepsy.
Quality control
The AMSTAR2 checklist showed a high score, indicating that this systematic review and meta-analysis is of a high standard (supplementary Appendix 3). The SIGN 50 checklist identified only 5 of the 32 studies to be of high quality and the Cochrane risk-of-bias assessment of the 19 controlled studies showed a high risk of bias in most domains for most of the studies (Fig. 7). A summary graph is presented as supplementary Appendix 5. Publication bias could not be identified using a funnel plot for stereotypy, as data from only three studies could be amalgamated into the meta-analysis. Funnel plot data showed no publication bias for aggression, which was further supported by Egger's test of publication bias (P = 0.213).Reference Egger, Smith, Schneider and Minder80 Although the funnel plot showed some publication bias for overall rate of challenging behaviour, this was not significant under Egger's test of publication bias (P = 0.734). There was no publication bias present for self-injurious behaviour (P = 0.307). Eleven articles reported receiving funding from external sources, one did not receive any funding and the rest (n = 22) did not declare the funding source.

Fig. 7 Cochrane risk-of-bias summary for the 19 controlled studies.
+, bias present; −, bias absent; ?, bias possible.
Discussion
We included 34 articles in our systematic review that met the eligibility criteria. These included 9 studies that compared the overall rate of challenging behaviour in an epilepsy group with a matched control group and another 10 that compared data with an unmatched control group. Compared with previous systematic reviews this review included data for a much higher number of participants (Table 4).
Table 4 Comparison of systematic reviews

In our review, of the total 19 controlled studies, 13Reference Deb, Thomas and Bright12, Reference Deb, Cowie and Richens36, Reference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Collacott39, Reference Deb40, Reference Matthews, Weston, Baxter, Felce and Kerr43–Reference Prasher45, Reference Turkistani47, Reference Fitzgerald, Matson and Barker50–Reference Blickwedel, Vickerstaff, Walker and Hassiotis53 did not show any significant intergroup difference in the overall rate of challenging behaviour, 3Reference Gillies, Espie and Montgomery38, Reference Chung and Cassidy42, Reference McGrother, Bhaumik, Thorp, Hauck, Branford and Watson48 showed a significantly higher rate of challenging behaviour in the epilepsy group and 3Reference Matson, Bamburg, Mayville and Khan41, Reference Espie, Watkins, Curtice, Espie, Duncan and Ryan46, Reference Pawar and Akuffo49 showed a higher rate in the non-epilepsy group. Of these three, oneReference Matson, Bamburg, Mayville and Khan41 was at a significant level and the level of significance for the other twoReference Espie, Watkins, Curtice, Espie, Duncan and Ryan46, Reference Pawar and Akuffo49 is not known.
It was possible to pool data from only 16 of the controlled studies for a meta-analysis. Meta-analysis of pooled data from these 16 studies did not show a significant intergroup difference in the overall rate of challenging behaviour but showed a high heterogeneity. However, after sensitivity analysis meta-analysis of pooled data from 10 studies showed a significantly higher rate of overall challenging behaviour in the epilepsy group compared with the non-epilepsy group. Meta-analysis of pooled data showed a statistically significant higher rate of aggression and self-injurious behaviour in the epilepsy group compared with the non-epilepsy group. However, no such intergroup difference emerged from the meta-analysis of pooled stereotype data.
A funnel plot and Egger's test of publication bias showed no publication bias among the included studies. According to both SIGN 50 and Cochrane risk-of-bias assessments most studies appeared to be of moderate to poor quality.
Interpretation of meta-analyses findings
Our finding (based on meta-analysis of pooled data from 10 studies) of a significant intergroup difference differs from that of other systematic reviews,Reference Blickwedel, Ali and Hassiotis19–Reference van Ool, Snoeijen-Schouwenaars, Schelhaas, Tan, Aldenkamp and Hendriksen21 which found no such difference. Our finding has to be interpreted with caution. First, when data from all available studies were pooled, no statistically significant intergroup difference emerged, although the heterogeneity among studies was very high. Second, even when the meta-analysis after the sensitivity analysis showed that the epilepsy group had a significantly higher rate of challenging behaviour, the effect size remained very small, so this may not be clinically significant. Third, different studies defined challenging behaviour in different ways. Some did not use any validated tool. Fourth, even when a validated scale was used, the total score was used to define challenging behaviour, which is not always valid. For example, a number of studies used the ABC-C total score, which is not valid.Reference Aman, Burrow and Wolford66 Fifth, many studies used an arbitrary cut-off score on behaviour rating scales to define challenging behaviour, and different studies used different scales and different cut-off scores. Thus, it is difficult to compare data among studies as it is difficult to know when data were pooled and whether all studies are describing the same challenging behaviour.
Deb & HunterReference Deb and Hunter61 hypothesised that it is possible that underlying brain damage (in adults with severe and profound intellectual disabilities) and psychosocial factors (in those with mild intellectual disabilities) are stronger determinants of challenging behaviour than the presence of epilepsy. Factors that affect behaviour in the presence of epilepsy are related to: (a) underlying brain damage, such as the location and severity of any deformity, tumour or abnormal electrical discharge in the brain; (b) epilepsy-related factors, such as the presence of certain epileptic syndromes and genetic syndromes that are prone to lead to more challenging behaviour; (c) seizure-related factors, such as the severity, type and frequency of seizures; (d) anti-epileptic medication-related factors, such as the adverse effects of certain anti-epileptics and drug–drug interactions; and (e) psychosocial factors, such as loss of occupation, financial problems, lack of support, and locus of control being outside the person so the person does not have any control over the timing of seizures.
Types of challenging behaviour
Many types of challenging behaviour were assessed in the included studies. The most common types that cause most concern and are most difficult to manage are aggression (verbal or physical aggression towards other people or property) and self-injurious behaviour. Pooled data showed a statistically significant higher rate of both aggression and self-injurious behaviour in the epilepsy group compared with the non-epilepsy group. It is worth remembering that, apart from epilepsy, many other medical and psychosocial factors influence these behaviours, including certain genetic syndromes that are known to be associated with aggression and self-injurious behaviour.Reference Deb81 Many of these syndromes also tend to predispose individuals to epilepsy (examples include Lesch–Nyhan and fragile-X syndromes).Reference Deb1 Therefore, it is difficult to draw any conclusion about the association between these specific challenging behaviours and epilepsy in isolation without considering all the other predisposing (e.g. genetic syndromes), precipitating (e.g. infection or anxiety) and perpetuating (e.g. inappropriate treatment and/or environment) factors for challenging behaviour in general. Pooled data did not show any significant intergroup difference in the rate of stereotyped behaviour. Only a small number of studies were involved in this meta-analysis and the heterogeneity level was high. Therefore, this finding has to be interpreted with caution.
The rates of other behaviours, such as inappropriate sexual behaviour, irritability, hyperactivity, verbal aggression, antisocial behaviours and lethargy, were reported in a very small number of studies showing equivocal findings. Therefore, it is difficult to draw any definitive conclusion about the association between these specific types of challenging behaviour and epilepsy. Another problem is that none of these specific behavioural types was defined using any standardised criteria (such as the Modified Overt Aggression Scale for rating aggression)Reference Deb, Deb, Faruqui, Bodani and Agrawal15, Reference Ratey and Gutheil82 but were based on single-item scoring.
Association with epilepsy variables
When different subgroups according to various epilepsy variables were compared for the rate of challenging behaviour no clear picture emerged. In three studiesReference Creaby, Warner, Jamil and Jawad54, Reference Deb and Hunter61, Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 the rate of challenging behaviour was significantly higher in those who had generalised seizures compared with those who had focal seizures, but in one studyReference Andrews, Everitt and Sander67 no significant difference was reported between these two groups. Given the small number of studies involving small numbers of participants in the subgroups, lack of matching of the groups, and different types of challenging behaviour rated in these studies, it is difficult to draw any definitive conclusion about any association between challenging behaviour and seizure type. Furthermore, DebReference Deb62 has shown that a high proportion of adults with intellectual disabilities who had a clinical diagnosis of primary generalised seizure showed focal epileptiform changes in their EEGs, thus raising the possibility that in many cases these generalised seizures are secondarily generalised from focal seizures.
Although three studiesReference Gillies, Espie and Montgomery38, Reference Deb and Hunter61, Reference van Ool, Snoeijen-Schouwenaars, Tan, Schelhaas, Aldenkamp and Hendriksen72 showed a significantly higher rate of challenging behaviour among those who had frequent seizures compared with those who had less frequent seizures, five studiesReference Espie, Pashley, Bonham, Sourindhrin and O'donovan37, Reference Turkistani47, Reference Creaby, Warner, Jamil and Jawad54, Reference Deb and Joyce55, Reference Collacott, Cooper, Branford and McGrother58 did not find any significant intergroup difference. Therefore, it is difficult to draw any definitive conclusion about the influence of seizure frequency on the rate of challenging behaviour in this population. One confounder is the way frequency was rated in different studies, which varied widely. One studyReference Deb and Hunter61 showed a significantly higher rate of challenging behaviour in those whose EEGs showed generalised epileptiform activities compared with those whose EEGs showed focal epileptiform changes. However, in another three studiesReference Blickwedel, Vickerstaff, Walker and Hassiotis53, Reference Deb62, Reference Andrews, Everitt and Sander67 no significant intergroup difference emerged between those with generalised as opposed to focal EEG changes. It is difficult to carry out an EEG for many adults with intellectual disabilities, particularly those who have severe and profound disability and also those who have challenging behaviour. EEG records are available for approximately 50–70% of adults with intellectual disabilities and 70–90% of these recordings are abnormal, although the abnormalities are not necessarily epileptiform in nature; rather, they present mostly as non-specific excess slow background activity.Reference Deb62
Association with anti-epileptic medication
No definite association was found in the rate of challenging behaviour and polypharmacy with anti-epileptic medications. One would expect the participants in the polypharmacy group to have more severe epilepsy and, therefore, possibly more challenging behaviour. However, it is possible that anti-epileptic polypharmacy made these participants more sedated, thus dampening down the expression of challenging behaviour. In some people anti-epileptics improve both epilepsy symptoms and behaviour. However, in others it can have an opposite effect, in that although the epilepsy improves, the behaviour deteriorates.Reference Deb, Bouras and Holt9 There may be many explanations for this paradoxical response. Old theories, such as forced normalisationReference Tellenbach83 or alternative psychosis,Reference Landolt84 may provide some explanation. However, a more practical explanation may be that medication side-effects make the behaviour worse in some, despite improving epilepsy symptoms.
Although certain anti-epileptics, such as sodium valproate (restricted use in women of child-bearing age because of major worry about its teratogenicity), carbamazepine and lamotrigine, are known to improve mental state and are used to treat psychiatric disorders such as bipolar disorder,Reference Goodwin, Haddad, Ferrier, Aronson, Barnes and Cipriani85 paradoxically some anti-epileptics are known to precipitate psychopathology, including challenging behaviour.Reference Brodie, Besag, Ettinger, Mula, Gobbi and Comai86
Although the evidence is not strong, the available data suggest that the following anti-epileptic medications are likely to have some association with aggression and other challenging behaviours: phenobarbital, topiramate, vigabatrin, perampanel, zonisamide, levetiracetam, clobazam, clonazepam and tiagabine. Among these perhaps levetiracetam (aggression or agitation in 13% of treated patients), perampanel (in 12% at 8 mg/day and 20% at 12 mg/day) and possibly topiramate (in 2–10%) showed the strongest evidence for precipitating challenging behaviour.Reference Brodie, Besag, Ettinger, Mula, Gobbi and Comai86 However, levetiracetam seems to improve behaviour in some adults with intellectual disabilities and worsen it in others.Reference Deb and Saini87 These anti-epileptic-related adverse effects may be more pronounced among adults with intellectual disabilities.Reference Shankar, Watkins, Alexander, Devapriam, Dolman and Hari5 However, it is clear that vast majority of those who receive these medications do not show any challenging behaviour. It is difficult to draw any definite conclusion from this review, as only one study reported the rate of challenging behaviour related to specific anti-epileptic drugs (59% of participants on carbamazepine, 55% on sodium valproate, 53% on phenytoin and 78% on lamotrigine monopharmacy showed challenging behaviour).
Although monopharmacy with anti-epileptic medication is desirable,88 polypharmacy with anti-epileptics is common in intellectual disability populations. Therefore, anti-epileptic drug–drug interaction are more likely, some of which may lead to challenging behaviour. Given that both antipsychotic and antidepressant medications are commonly prescribed among adults with intellectual disabilities,Reference Deb and Singh89 their interaction with anti-epileptics must be considered in any assessment of challenging behaviour. Also, both antipsychotics and antidepressants are likely to lower seizure threshold (particularly the older generation ones and at a high dose), which may precipitate more seizures and may lead to challenging behaviour.
Subgroup comparisons do not provide adequate power to detect clinically significant difference because of the small numbers involved in each subgroup and the lack of a control group. Also, in the subgroups there is no consistency in the types of challenging behaviour described, as some studies provided the rate of overall challenging behaviour, but others reported the rates of different types of challenging behaviour, such as aggression, self-injurious behaviour and stereotypy, making it difficult to amalgamate data from different studies. It will be necessary to conduct a much larger randomised controlled trial to recruit a reasonable number of participants in each subgroup to provide adequate power to detect clinically significant intergroup differences.
Clinical significance of the findings
Having epilepsy can restrict social activities and wider social integration. A careful risk assessment is necessary to balance independence/quality of life and seizure-related risks (e.g. travelling alone, taking a bath, seizure-related injuries, unpredictability of the timing of seizures, SUDEP). The risk assessment should be part of the person's overall person-centred support plan and should be monitored and reviewed regularly with the person, their family/caregivers, other relevant professionals and the multidisciplinary team. One has to remember to mitigate against the impact on the family/caregivers. It is also important to remember that, apart from epilepsy, many circumstances, such as medical, psychological, social and environmental factors, affect the behaviour of someone with intellectual disabilities, and a full multidisciplinary person-centred assessment is required to develop an appropriate formulation for the management of challenging behaviour, including psychosocial interventions.Reference Deb, Kwok, Bertelli, Salvador-Carulla, Bradley and Torr10 Support staff, and the person and their family/carers, need to be informed of the risk factors (including SUDEP) and prognosis.Reference Young, Shankar, Palmer, Craig, Hargreaves and McLean90–Reference Devinsky92 This also highlights the requirement for regular health checks for all adults with intellectual disabilities, as highlighted in a recent NHS England publication.93
Strengths
Our review received a high rating on the AMSTAR 2 quality control checklist for systematic reviews, as we complied with all of its requirements (supplementary Appendix 3). We carried out meta-analyses that were not done in any previous systematic reviews. We included a comprehensive Cochrane risk-of-bias table, which was not done by any of the previous systematic reviews. We included a much higher number of articles covering data from a much larger number of participants compared with the previous systematic reviews. We have registered our review with a well-established database, PROSPERO, so that our protocol is available for public scrutiny. This was not done by the previous reviews. We also carried out a very extensive hand-search of journals in the field of intellectual disability and epilepsy, along with stringent cross-referencing.
Weaknesses
We searched for articles in English only. We excluded the grey literature and conference abstracts, as we felt it would be difficult to apply our eligibility criteria and risk-of-bias assessment on the basis of abstracts only. Although we used a stringent method for literature search, it is still possible that we missed some relevant articles. Our analysis showed conflicting evidence, in that the meta-analysis of pooled data from a larger number of studies did not show a significant intergroup difference, whereas pooled data from a smaller number of studies after sensitivity analysis showed a significant difference. Although we amalgamated data where possible to carry out a number of meta-analyses, the heterogeneity among studies remains high. It is difficult to amalgamate data from studies that used such diverse methodologies and defined challenging behaviour in so many different ways. Therefore, our findings must be interpreted with caution, as a lot of confounders could not be controlled for. However, to counteract the problem with study heterogeneity we used sensitivity analyses. Ideally, we should have used raw data for the meta-analysis, but this was not possible. Also, by log-transforming some data we may have lost some power in the meta-analysis.
Research implications
Even though the meta-analysis of pooled data from a smaller number of studies after sensitivity analysis showed a significantly higher rate of challenging behaviour in the epilepsy group, the effect sizes are small, which may not be clinically significant. Also, there are major methodological flaws (highlighted by Cochrane risk-of-bias and SIGN 50 assessments) in the included studies. There is therefore a need for large-scale properly controlled studies. The included studies primarily concentrated on inter-ictal challenging behaviour. However, peri-ictally some people may show aggression, which is not goal directed but inadvertently may injure others. This might also be studied in further research.
Supplementary material
Supplementary material is available online at http://doi.org/10.1192/bjo.2020.96.
Data availability
Data availability is not applicable to this article as no new data were created or analysed in this study.
Author contribution
B.A.B. and S.D. conceptualised and designed the study. B.A.B. carried out the literature search. B.A.B. and B.L. screened bibliographies and extracted data and completed risk-of-bias checklist. B.L. carried out meta-analysis. All authors contributed to manuscript writing and authorised the final version of the manuscript.
Funding
B.L. is funded by the UK's National Institute of Health Research (NIHR) Research for Patient Benefit (RfPB) Programme (grant PBPG-0817-20010). The Imperial Biomedical Research Centre Facility, which is funded by the NIHR, has provided support for the study. The views expressed in this article are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health, UK.
Declaration of interest
None.
ICMJE forms are in the supplementary material, available online at http://doi.org/10.1192/bjo.2020.96.














eLetters
No eLetters have been published for this article.