Percutaneous closure of perimembranous ventricular septal defect is considered a safe and effective alternative to surgery in selected cases, Reference Saurav, Kaushik and Mahesh Alla1–Reference Pillai, Rangasamy and Balasubramonian8 with a similar procedural success rate, outcome and reduced hospital stay. Reference Saurav, Kaushik and Mahesh Alla1 Despite initial concerns regarding the use of the Amplatzer Membranous ventricular septal defect occluder (AGA Medical Corp., Minnesota, US) due to the occurrence of atrioventricular block, Reference Fu, Bass and Amin9 many different devices have been used off-label with promising results. Reference Haddad, Daou and Saliba2–Reference Hua, Aquino and Owada4 Patients with perimembranous ventricular septal defects and inlet to outlet extension are a real challenge for the interventional cardiologist because of the large size and the crescentic shape of such defects. We report two cases of percutaneous closure of large perimembranous ventricular septal defects with inlet to outlet extension using an Amplatzer Vascular Plug-II (AVP-II, St Jude Medical, Minnesota, US).
Case report 1
A 2-year-old, 12 kg girl was followed for a large perimembranous ventricular septal defect, with failure to thrive and mild degree of cardiac failure necessitating diuretics since infancy. Her transthoracic echocardiogram showed a large high velocity (4 m/s through continuous Doppler interrogation) defect with inlet to outlet extension and important left ventricular volume overload. She was discussed in our Multidisciplinary Team Meeting, and there was agreement for percutaneous approach. She was therefore referred for semi-elective percutaneous closure of her ventricular septal defect. Under general anaesthesia in the catheterisation laboratory, transthoracic and transesophageal echocardiography showed a large (10 mm diameter) perimembranous ventricular septal defect which was partially covered by tricuspid valve tissue (Fig 1a, b, and c). After ultrasound-guided puncture of the right femoral artery, a 4Fr pigtail catheter was advanced to the left ventricle and a left ventricle angiogram was performed (Fig 1d). A 4Fr multipurpose catheter (MPA2, Cordis, Hamburg, Germany) was successfully used to cross the ventricular septal defect over a 0.035’’ Terumo wire (Terumo Medical, Somerset, NJ), from the left ventricle towards the right ventricular. After crossing the defect, the wire directly went through the right ventricle outflow till the distal right pulmonary artery (Fig 2a and b). After pulling back the Terumo wire over the multipurpose catheter and advancing the catheter wire towards the apex of the right ventricle, the Terumo wire was exchanged with a semi-rigid 0.035'' diagnostic “J” tip guidewire (Medline International B.V., Arnhem, Netherlands). The multipurpose catheter was removed, and a 5 Fr Cook flexor sheath (Cook Medical; Bloomington, U.S.A.) was then advanced over the wire. A 12 mm Amplatzer Vascular Plug-II was advanced through the long sheath and positioned with the distal and middle disk into the right ventricular side of the defect, whereas the proximal disk was left on the left ventricular side of the defect. The transesophageal echocardiography demonstrated almost complete occlusion of the defect with alignment of the right ventricular side of the device almost parallel to the interventricular septum. There was mild tricuspid regurgitation and no significant aortic regurgitation. The electrocardiogram remained in sinus rhythm with no conduction anomaly. The device was released after confirmation of its stability with transesophageal and transthoracic echocardiogram (Fig 3a). Post-procedure echocardiography in the ward confirmed the excellent procedural result with trivial residual shunt (Fig 3b and c). The child was discharged the following day on aspirin. Short-term follow-up at 3 months after the procedure revealed no residual shunt, normal function of the aortic valve, a stable mild tricuspid regurgitation, and no subsequent conduction anomaly on the electrocardiogram. A 24-hour Holter monitoring performed 6 weeks after the procedure did not show any arrhythmia or conduction anomalies.
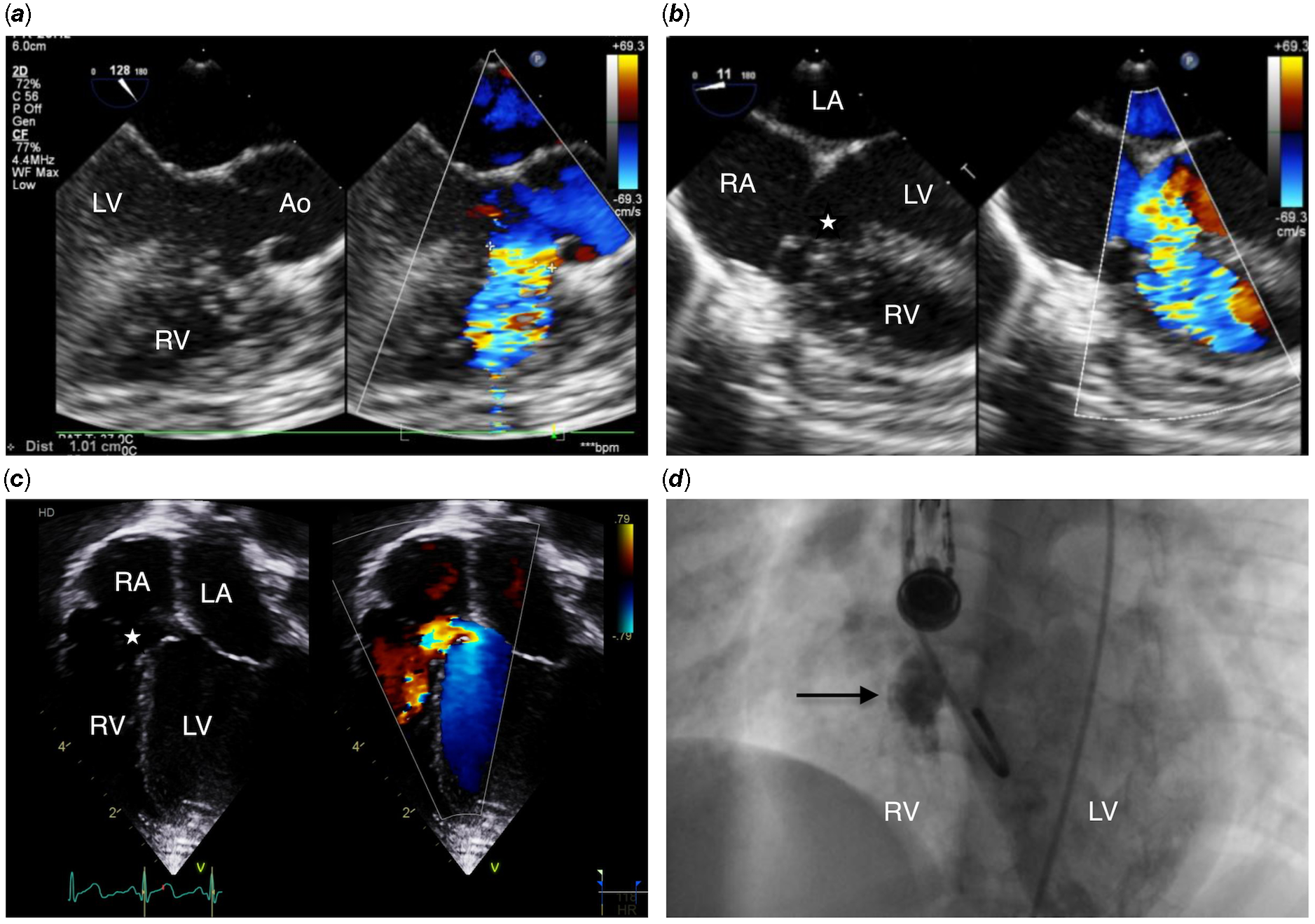
Figure 1. ( a ) Transesophageal echocardiogram demonstrates a 10 mm large perimembranous ventricular septal defect with significant malalignment of the ventricular septum and no aortic rim. In ( b ) and ( c ), there is accessory tricuspid valve tissue (white star) and inlet extension, with no rim toward the septal leaflet of the tricuspid valve, with large left to right shunt. In ( d ), a left ventricular angiogram confirmed a large perimembranous ventricular septal defect measured at 10 mm (black arrow). Ao = Aorta; LV = Left ventricle; RV = Right ventricle; RA = Right atrium; LA = Left atrium.

Figure 2. ( a ) A 0.035'' Terumo guidewire over a 4Fr multipurpose catheter was easily advanced across the defect into the right pulmonary artery. In ( b ), transesophageal echocardiogram short-axis view confirmed the outlet extension of the defect. AoV = Aortic valve; RV = Right ventricle.
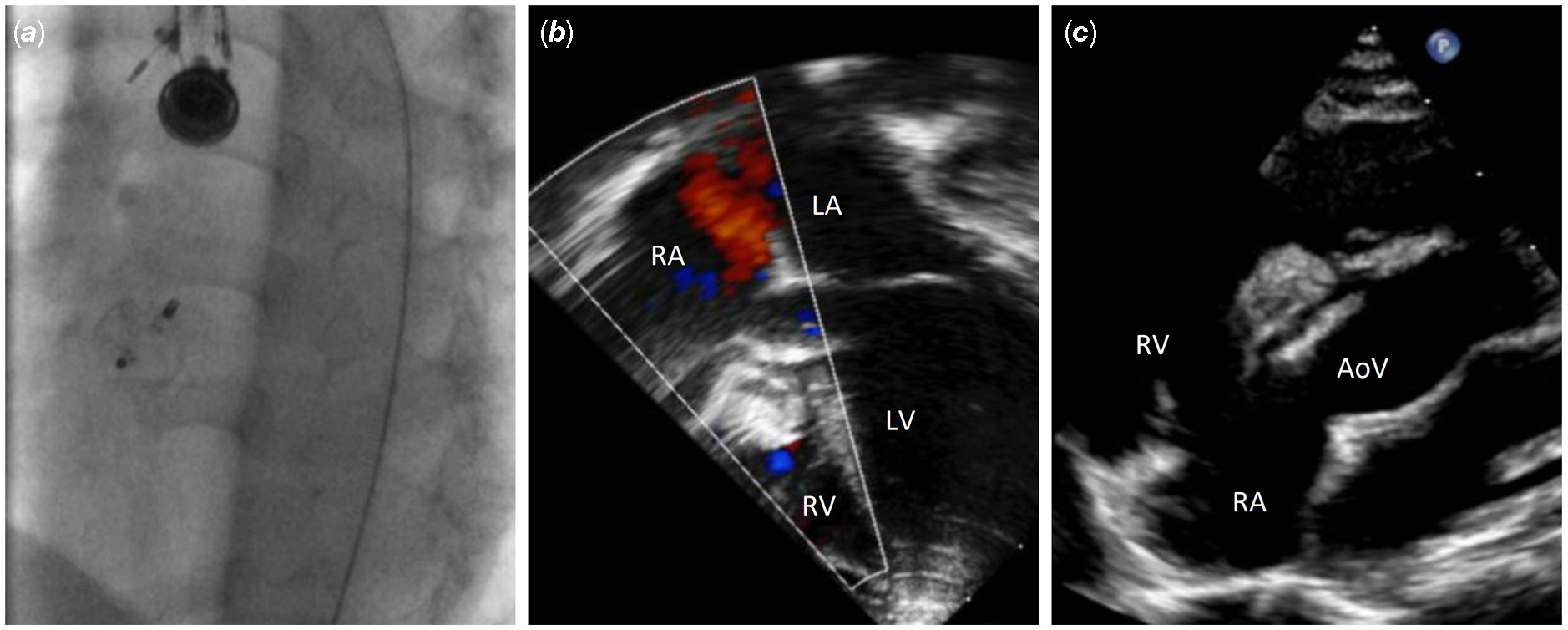
Figure 3. ( a ) A 12 mm AVP-II device was successfully positioned and released. In ( b ) and ( c ), transthoracic echocardiogram showed almost no residual shunt and normal opening of the tricuspid valve despite the protrusion of the AVP-II on the RV side. AVP-II = Amplatzer Vascular Plug-II; AoV = Aortic Valve; LV = Left ventricle; RV = Right ventricle; RA = Right atrium; LA = Left atrium.
Case report 2
A 7-year-old, 22 kg boy was under follow-up for a perimembranous ventricular septal defect partly covered by tricuspid valve tissue. He was clinically well but his mother reported progressively reduced exercise tolerance. On transthoracic echocardiogram, there was significant dilatation of the left ventricle with increased left ventricle end-diastolic diameter (Z score + 3.5) (Fig 4a). A decision to close his defect percutaneously was made after Multidisciplinary Team discussion. The transthoracic and transesophageal echocardiography and the left ventricle angiogram showed a large perimembranous ventricular septal defect (16 mm diameter) extending from the inlet to the outlet (Fig 4b, c and d). A retrograde approach through the aorta and the left ventricle was used with the same catheter and wire than in Case report 1, to cross the defect, and a 7 Fr Cook flexor sheath (Cook Medical; Bloomington, U.S.A.) was advanced into the right ventricle. Initially, a 16 mm Amplatzer Vascular Plug-II was positioned but the result was not satisfactory, with mild residual shunt even after repositioning the device. A decision was taken to upsize to an 18 mm Amplatzer Vascular Plug-II that was positioned and successfully released with optimal result, no conduction abnormalities on electrocardiogram and no residual shunt on the transesophageal and transthoracic echocardiogram (Fig. 5). Short-term follow-up at 6 months confirmed the excellent result of the procedure with no residual shunt and normal function of both aortic and tricuspid valves (Video 1). Follow-up Holter and electrocardiogram at 6 weeks did not show any arrhythmia or conduction anomalies.
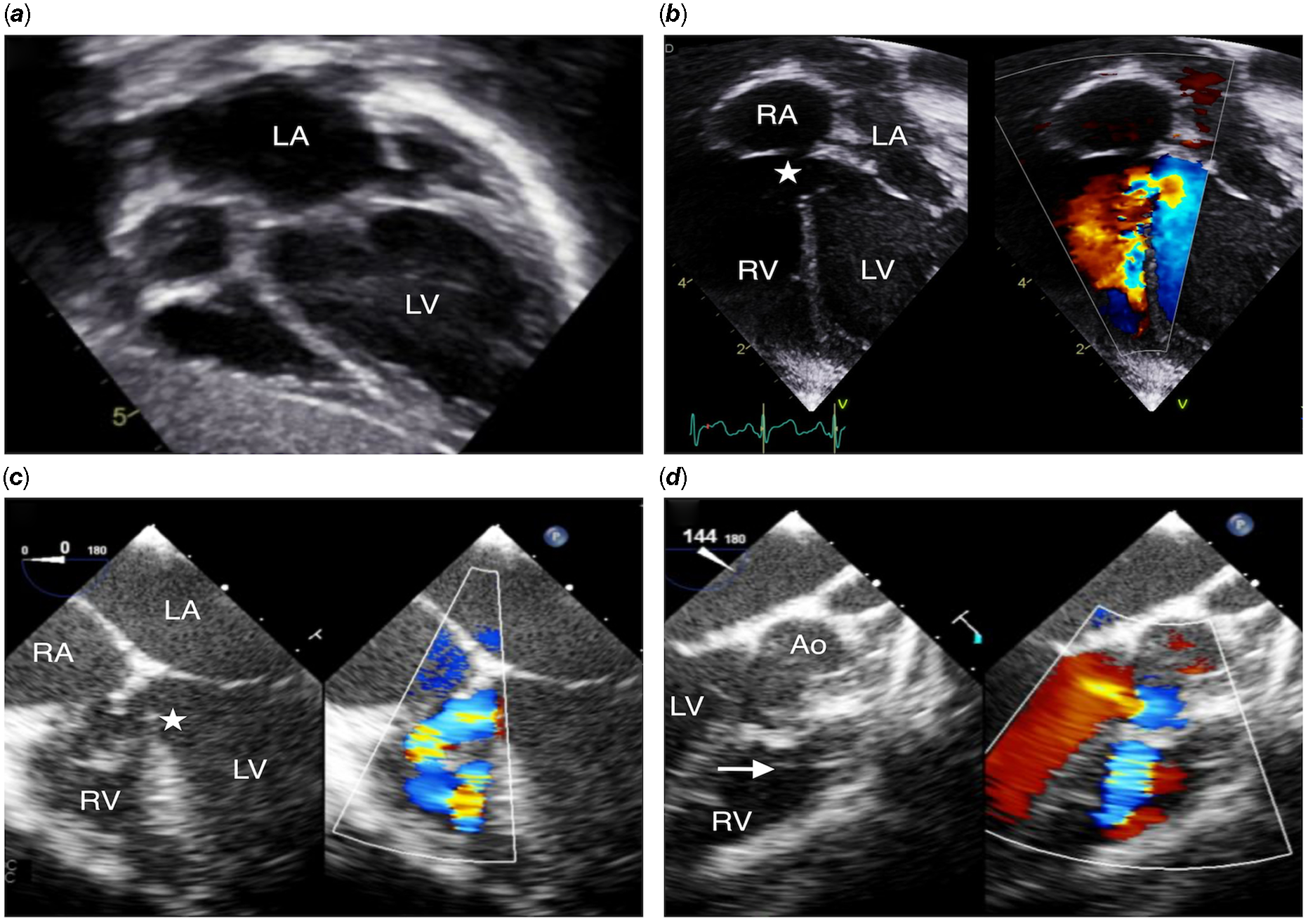
Figure 4. ( a ) shows severely dilated left atrium and left ventricle on transthoracic echocardiogram. In ( b ), ( c ), and ( d ), transthoracic and transesophageal echocardiogram demonstrated a large perimembranous ventricular septal defect (16 mm) with inlet (white star) to outlet extension (white arrow showing the pulmonary valve), malalignment of the ventricular septum, and almost no aortic rim as well as no rim towards the septal leaflet of the tricuspid valve, with large left to right shunt. Ao = Aorta; LV = Left ventricle; RV = Right ventricle; RA = Right atrium; LA = Left atrium.
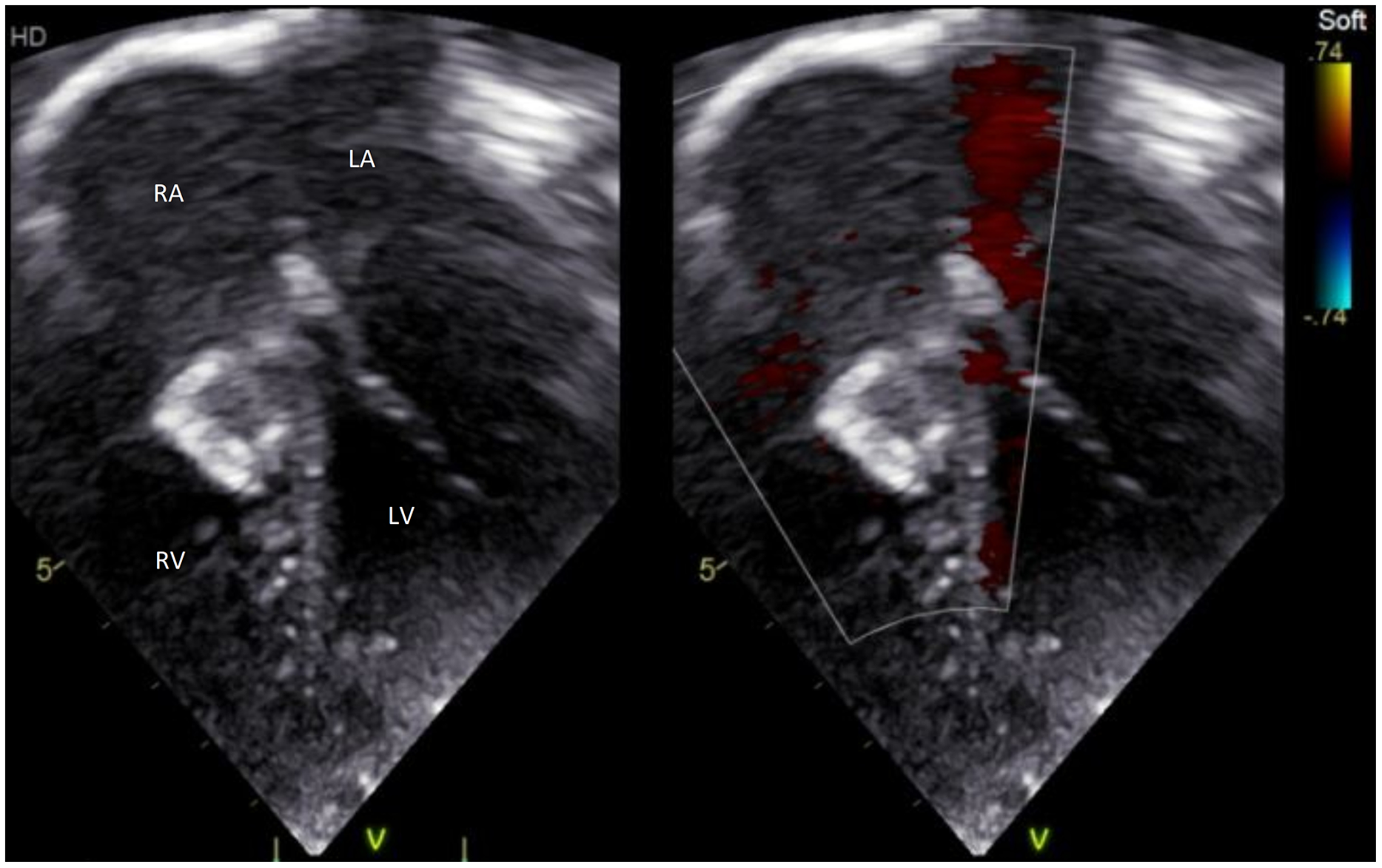
Figure 5. An 18 mm AVP-II device was successfully implanted with excellent result and no residual shunt. AVP-II = Amplatzer Vascular Plug-II; LV = Left ventricle; RV = Right ventricle; RA = Right atrium; LA = Left atrium.
Discussion
Numerous devices have been used in the last decades for transcatheter closure of perimembranous ventricular septal defects, starting from the Amplatzer Membranous ventricular septal defect occluder, Reference Fu, Bass and Amin9 up to the off-label use of PDA occluders and vascular plugs. Reference Haddad, Daou and Saliba2–Reference Hua, Aquino and Owada4,Reference Pillai, Rangasamy and Balasubramonian8 Our two cases of transcatheter closure with inlet to outlet extension of large perimembranous ventricular septal defects were successfully closed with the Amplatzer Vascular Plug-II.
The Amplatzer Vascular Plug-II was initially designed for arterial and venous embolisation in the peripheral vasculature. It has peculiar features that make it interesting for the closure of large perimembranous ventricular septal defect. First, the relatively low profile allows a technically easy implantation of the device through a retrograde approach from the femoral artery. The defect is generally easily crossed under transesophageal echocardiography guidance. The largest part of the device is usually positioned on the right ventricular side of the septum, leaving only the proximal disc into the left ventricle outflow, which allows closure of defects with almost no aortic rim. Second, the longitudinal shape of the Amplatzer Vascular Plug-II allows the device to align almost parallel to the ventricular septum allowing occlusion of the defect despite its crescentic shape. Because of this orientation as well as the softness of the device, the function of the tricuspid valve is usually preserved. Finally, we speculate that the fabric-less structure and softness of the Amplatzer Vascular Plug-II as well as the absence of true retention disks with a memory shape reduces the burden of complications such as atrioventricular block. This also demonstrates that the absence of fabric does not prevent complete occlusion of the defects.
Conclusion
In conclusion, the Amplatzer Vascular Plug-II allows closure of large perimembranous ventricular septal defect with inlet to outlet extension. More experience and longer-term follow-up are needed to confirm those promising results.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122003407
Authors’ contributions
Enrico Piccinelli, Carles Bautista-Rodriguez, and Alain Fraisse contributed in the drafting of the manuscript and final approval of the manuscript submitted.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
Alain Fraisse is consultant and proctor for Medtronic, Abbott, and for Occlutech. The other authors have nothing to declare.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committee.








