Contamination of shellfish by paralytic shellfish poisoning (PSP) toxins poses an economic threat to shellfish farmers. As contaminated shellfish cannot be harvested for long periods of time, it would be very useful to develop processes to optimise and shorten their detoxification. In this study, Pacific oysters Crassostrea gigas were first experimentally contaminated over a period of 13 days with a continuous flow of toxic Alexandrium minutum cultures at concentrations ranging from 150 to 200 cell ml-1 (toxin content after 13 days of contamination 438 $\mu $ 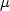 g STX equiv. 100 g−1 wet weight). Then, two different detoxification treatments were tested and showed detoxification rates greater than those observed in coastal environments. The first treatment consisted of feeding oysters on Skeletonema costatum, at a concentration of 2000 cell ml−1 to speed up detoxification rates. The second detoxification method used the same Skeletonema costatum diet, supplemented with silt particles at a concentration of 20 mg L−1. A control was also set up by placing contaminated oysters in seawater with no additional algal food. The detoxification experiment lasted 8 days. Toxin contents were analysed by liquid chromatography with fluorescence detection (LC-FD). The S. costatum diet significantly reduced the time needed for oysters to reach the sanitary threshold (80 $\mu $
g STX equiv. 100 g−1 wet weight). Then, two different detoxification treatments were tested and showed detoxification rates greater than those observed in coastal environments. The first treatment consisted of feeding oysters on Skeletonema costatum, at a concentration of 2000 cell ml−1 to speed up detoxification rates. The second detoxification method used the same Skeletonema costatum diet, supplemented with silt particles at a concentration of 20 mg L−1. A control was also set up by placing contaminated oysters in seawater with no additional algal food. The detoxification experiment lasted 8 days. Toxin contents were analysed by liquid chromatography with fluorescence detection (LC-FD). The S. costatum diet significantly reduced the time needed for oysters to reach the sanitary threshold (80 $\mu $ 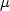 g STX equiv. 100 g−1 wet weight), but no effect of the silt supplement could be demonstrated conclusively. These different detoxification methods did not influence toxin biotransformations as observed in oyster tissues, i.e. epimerisation and decarbamoylation of gonyautoxins 2 and 3.
g STX equiv. 100 g−1 wet weight), but no effect of the silt supplement could be demonstrated conclusively. These different detoxification methods did not influence toxin biotransformations as observed in oyster tissues, i.e. epimerisation and decarbamoylation of gonyautoxins 2 and 3.