Introduction
Island ecosystems are often more vulnerable to biological invasions than continental ecosystems (Chown & Lee Reference Chown, Lee, Gillespie and Clague2009). This is particularly relevant in the sub-Antarctic, where the geographical isolation and cold oceanic climate have resulted in species-poor terrestrial biodiversity and detritus-based food webs lacking predators (Chown & Convey Reference Chown and Convey2016). As a result, most sub-Antarctic (and Antarctic) communities are primarily structured by abiotic factors (Hogg et al. Reference Hogg, Craig Cary, Convey, Newsham, O’Donnell and Adams2006) and have a relatively simple trophic structure. In a biological invasion context, this may leave native species more vulnerable to the emergence of invaders relative to species from more taxonomically diversified regions (Diamond & Case Reference Diamond and Case1986, Carthey & Banks Reference Carthey and Banks2014). Additionally, the species-poor nature of sub-Antarctic ecosystems means that invaders outnumber native species in many taxonomic groups (Jones et al. Reference Jones, Chown, Ryan, Gremmen and Gaston2003, Frenot et al. Reference Frenot, Chown, Whinam, Selkirk, Convey, Skotnicki and Bergstrom2005). This can also mean that introduced species have adaptations or morphological differences allowing them to exploit unoccupied niches or to more effectively exploit resources, such as the morphologically unique invader Porcellio scaber (Latreille, 1804) introduced to the sub-Antarctic (Martin et al. Reference Martin, Aerts, Convey and Bokhorst2023). The effects of competition and predation may thus significantly threaten native sub-Antarctic biota, which lack sufficient defences against novel weapons and predators (Callaway & Ridenour Reference Callaway and Ridenour2004, Daly et al. Reference Daly, Chabrerie, Massol, Facon, Hess and Tasiemski2023b). This has been well illustrated with albatrosses, which evolved to breed on islands without predators and now lack effective behavioural responses to ward off predatory invasive mice, leading to significant chick and occasional adult mortality (Connan et al. Reference Connan, Jones, Risi, Smyth, Oppel and Perold2024). Less obvious are the impacts of invasive predatory insects, which may have similar potential to decimate native populations of rare and endemic species (Lebouvier et al. Reference Lebouvier, Lambret, Garnier, Convey, Frenot, Vernon and Renault2020). However, the severity of these impacts remain poorly understood (Chown & Convey Reference Chown and Convey2016).
Invasive generalist insect predators pose significant problems for invaded ecosystems because of their tendency to reach higher population densities than native predators, their frequent consumption of both native herbivores and predators and their potential competition with native predators (Crowder & Snyder Reference Crowder and Snyder2010). Invasive predatory ground beetles can have wide-ranging effects in their novel ranges, probably owing to the plasticity and flexibility in their diets, habitats and behaviours (Evans et al. Reference Evans, Soares and Yasuda2011). The presence of invasive carabids, such as Trechus obtusus Erichson, 1837 and Pterostichus melanarius Illiger, 1798, has been linked with declines in native carabids in their introduced ranges, although in the case of P. melanarius these effects are habitat-specific (Liebherr & Krushelnycky Reference Liebherr and Krushelnycky2007, Blubaugh et al. Reference Blubaugh, Asplund, Judson and Snyder2021, Busch et al. Reference Busch, Wham and Tooker2021).
As the effects of invasive predators can also interact with other processes to amplify or accelerate biodiversity declines, understanding their ecological interactions in their novel ranges is key to understanding their impacts. Doherty et al. (Reference Doherty, Dickman, Nimmo and Ritchie2015) identify three main pathways through which invasive predators synergize with disturbance to increase biodiversity declines. The first is that ecological disturbance can improve habitat quality or availability, increasing invasive predator abundance and decreasing the abundance of its native prey (Doherty et al. Reference Doherty, Dickman, Nimmo and Ritchie2015). Second, disturbance can affect the per capita impact of the predator on its prey, leading to prey declines despite stable predator populations (Doherty et al. Reference Doherty, Dickman, Nimmo and Ritchie2015). For example, habitat change following fire can reduce prey refuge availability, leading to a functional change in the predator-prey relationship and higher rates of predation (Conner et al. Reference Conner, Castleberry and Derrick2011, Doherty et al. Reference Doherty, Dickman, Nimmo and Ritchie2015, Reference Doherty, Geary, Jolly, Macdonald, Miritis and Watchorn2022). Finally, the effects of invasive predators can also interact with disturbances that reduce prey abundance, leading to disproportionately negative impacts of the invasive predator that compound prey decline in a similar way to the Allee effect (Allee Reference Allee1938, Doherty et al. Reference Doherty, Dickman, Nimmo and Ritchie2015).
In the sub-Antarctic, there are appreciable numbers of invertebrate invaders, with numerous predators representing diverse guilds from flatworms (Houghton et al. Reference Houghton, Terauds and Shaw2022) to wasps (Lee & Chown Reference Lee and Chown2016) to carabids (Ernsting et al. Reference Ernsting, Block, MacAlister and Todd1995, Brandjes et al. Reference Brandjes, Block and Ernsting1999, Ouisse et al. Reference Ouisse, Laparie, Lebouvier and Renault2017). Worryingly, all of these predators have generally exhibited high dispersal abilities and have rapidly expanded beyond their initial introduction sites. However, their impacts are as diverse as their taxonomies and the communities they invade. For example, in its introduced range the invasive wasp Aphidius matricariae Haliday, 1834 is only known to prey on the invasive aphid Rhopalosiphum padi Linnaeus, 1758 (Lee & Chown Reference Lee and Chown2016) and thus probably has a smaller impact on native invertebrates than more generalist predators such as carabids. Multiple sub-Antarctic islands have been colonized by predatory carabid beetles, such as Merizodus soledadinus Guerin-Meneville, 1930 (Convey et al. Reference Convey, Key, Key, Belchier and Waller2011, Ouisse et al. Reference Ouisse, Laparie, Lebouvier and Renault2017) and Trechisibus antarcticus Dejean, 1831 (Ernsting et al. Reference Ernsting, Block, MacAlister and Todd1995, Brandjes et al. Reference Brandjes, Block and Ernsting1999, Ouisse et al. Reference Ouisse, Laparie, Lebouvier and Renault2017). However, even generalist species can have feeding preferences that influence their trophic interactions and population dynamics and therefore their impacts as invaders. For example, in the South Georgia Islands (Ernsting et al. Reference Ernsting, Block, MacAlister and Todd1995), the introduction of T. antarcticus has led to dramatic reductions in the perimylopid beetle Hydromedion sparsutum Müller, 1884, while M. soledadinus has had a similar effect on the sphaerocerid wingless fly Anatalanta aptera Eaton, 1875 in the Kerguelen Archipelago (Chevrier et al. Reference Chevrier, Vernon, Frenot, Battaglia, Valencia and Walton1997, Ouisse et al. Reference Ouisse, Laparie, Lebouvier and Renault2017).
As these invaders continue to expand and their favoured prey consequently decline in abundance, other species may become at risk. In the case of M. soledadinus, which was first observed in the Kerguelen Archipelago in 1939 (Jeannel Reference Jeannel1940) at Port-Couvreux (49°16’50.0”S, 69°41’29.0”E), the species is now widely distributed and is actively expanding its range (Lebouvier et al. Reference Lebouvier, Lambret, Garnier, Convey, Frenot, Vernon and Renault2020). M. soledadinus is thought to have been introduced to the Kerguelen Archipelago from the Falkland Islands, where it is common, through the import of sheep and their fodder in 1912 (Lebouvier et al. Reference Lebouvier, Lambret, Garnier, Convey, Frenot, Vernon and Renault2020). Since then, this flightless predator has spread, mainly in coastal habitats, and colonized many other sites, leading to widespread declines in A. aptera (Chevrier et al. Reference Chevrier, Vernon, Frenot, Battaglia, Valencia and Walton1997, Lebouvier et al. Reference Lebouvier, Lambret, Garnier, Convey, Frenot, Vernon and Renault2020). Despite considerable study of the morphology and physiology of this species in its invasive range (Laparie & Renault Reference Laparie and Renault2016, Ouisse et al. Reference Ouisse, Laparie, Lebouvier and Renault2017, Reference Ouisse, Day, Laville, Hendrickx, Convey and Renault2020, Engell Dahl et al. Reference Engell Dahl, Bertrand, Pierre, Curtit, Pillard and Tasiemski2019), published data regarding its diet and feeding behaviour are lacking. This has inhibited understanding of its current impacts, as well as predictions about its future range and impacts (Géron et al. Reference Géron, Cuthbert, Hotte and Renault2023). In order to predict the future trajectories and impacts of invasive predators, it is crucial to understand their predatory behaviours and dietary breadth.
To address this gap, we conducted several experiments under controlled conditions aiming to characterize M. soledadinus’ predatory nature and potential in the Kerguelen Archipelago. Specifically, we examined its density-dependent feeding behaviour, prey choices and responses to olfactory cues. We tested the role of predator density and prey identity on attack behaviour. As M. soledadinus has been observed in the field tackling larger larvae as a group, we hypothesized that greater predator density would decrease the time to attack and increase the diversity of prey attacked, as group dynamics may encourage predation. Additionally, we hypothesized that all dipteran larvae would be consumed by M. soledadinus because of its known predation on A. aptera larvae and because it is thought to be responsible for population declines in this species (Lebouvier et al. Reference Lebouvier, Lambret, Garnier, Convey, Frenot, Vernon and Renault2020). As little is known regarding the foraging behaviour of M. soledadinus, we also tested the role of olfactory cues characteristic of terrestrial and coastal habitats and potential prey to assess whether M. soledadinus uses odours to aid in orientating itself. In the case that this sensory input is important for this species, as it is known to be for other carabids (Tréfás et al. Reference Tréfás, Canning, McKinlay, Armstrong and Bujáki2001, Kulkarni et al. Reference Kulkarni, Dosdall, Spence and Willenborg2017), we hypothesized that it would prefer odours from plants characteristic of habitats where it is frequently found in the Kerguelen Archipelago to unloaded arms (lacking scented material) of the experimental apparatus. Similarly, as a voracious predator, we also hypothesized that it would be attracted by prey odour in comparison to unscented arms.
Methods
Study species and native biodiversity
M. soledadinus (Coleoptera, Carabidae) is a year-round active species in the Kerguelen Archipelago. It can be found primarily under stones and tide drift lines in coastal areas. In the present work, adults were hand-collected in Port-aux-Français (70°12’59.76”E, 49°21’0.00”S) and then maintained under controlled conditions at 8 ± 1°C. Batches of 100 insects were held for 1 week in plastic boxes (11.5 × 8.5 × 5.0 cm, L × l × h) and were supplied with water but without food. A preliminary study determined that food deprivation periods as long as 6 weeks did not affect the activity patterns of the species, and similar conclusions were reported in previous investigations (Laparie et al. Reference Laparie, Larvor, Frenot and Renault2012, Renault et al. Reference Renault, Leclerc, Colleu, Boutet, Hotte and Colinet2022).
The terrestrial macroinvertebrate community in the Kerguelen Archipelago is species-poor, consisting of only 25 native species, 75% of which belong to the orders Coleoptera and Diptera (Hullé & Vernon Reference Hullé and Vernon2021a,Reference Hullé and Vernonb). The 11 native Coleoptera are mostly represented by weevils, but they also include Meropathus chuni Enderlein, 1901 (Hydraenidae) and staphylinid Leptusa atriceps C.O. Waterhouse, 1875 (Hullé & Vernon Reference Hullé and Vernon2021a,Reference Hullé and Vernonb). Native Diptera are less numerous and are outnumbered 11 to 7 by introduced congeners. Native Diptera are mostly flightless and belong to five different families. The remaining seven native macroinvertebrates consist of one earthworm (Microscolex kerguelarum Grube, 1877), two spiders (Myro kerguelenensis kerguelenensis Pickard-Cambridge, 1876 and Neomaso antarcticus Hickman, 1939), one flightless parasitoid wasp (Kleidotoma icarus Quinlan, 1964), two flightless moths (the larger Pringleophaga kerguelensis Enderlein, 1905 and the smaller Embryonopsis halticella Eaton, 1875) and one Psocoptera (Antarctopsocus daviesi Badonnel, 1970; Hullé & Vernon 2021a,b). Of the 31 established invasive species, the majority are composed of Araneae (5), Coleoptera (4), Diptera (11), Hempitera (6) and Crassiclitellata (3), in addition to single members from the orders Psocoptera and Thysanoptera (Hullé & Vernon Reference Hullé and Vernon2021a,Reference Hullé and Vernonb).
Merizodus soledadinus density experiment
In a first part of the study, we investigated the importance of density on the predatory behaviour of M. soledadinus, as lone individuals may not be capable of taking on all prey species effectively. To do this, we presented different prey types to single M. soledadinus individuals and groups of 10 individuals (see Table I for number of trials per species). The prey used were larval dipteran Calycopteryx moseleyi Eaton, 1875, annelid M. kerguelarum Grube, 1877 and larval lepidopteran P. kerguelensis Enderlein, 1905. As with the prey choice experiment, predators fasted for 1 week prior to the start of the experiment. The fasting period and experiments were conducted at 8°C by holding adult M. soledadinus individually in Petri dishes covered with filter paper on their bases and moistened with tap water.
Table I. Different prey species (and corresponding life stages) presented to lone Merizodus soledadinus individuals or groups of 10 M. soledadinus individuals for feeding trials.

A = adult; L = larva.
The experiments were conducted in Petri dishes (diameter: 9 cm). For trials with a single M. soledadinus individual, the invertebrates were initially observed for a period of 1 h, with behavioural observations taken after 1, 30 and 60 min. The time of first contact, defined as the time at which the antennae or mandibles of one adult M. soledadinus first touched the prey, was also noted when it occurred within this first hour. Each Petri dish was then left for a period of 24 h, at which point a final observation of the remaining prey was taken. For trials with 10 M. soledadinus individuals, the invertebrates were observed for 1 h or until nothing remained of the prey, if that occurred before the hour had elapsed. We recorded the times of first contact between one, two, three and four or more M. soledadinus individuals and the prey.
To determine whether prey type or predator abundance influenced the initiation of predatory behaviour, we compared the time to first contact with prey across prey type and predator abundance as well as prey mortality after 5 h. For the time to first contact, we used a Kruskal-Wallis test as the data were not normally distributed (W = 0.754, P < 0.05). We were interested in determining whether the presence of other adult individuals reduced the decision time to attack, as has been shown in other species (Gamberale-Stille Reference Gamberale-Stille2000). Similarly, we were interested in how decision time differed for M. soledadinus depending on prey type. We determined this using a Dunn’s test to examine pairwise differences. To compare prey mortality across groups, we tested for significant differences using a two-sided Fisher’s exact test.
Prey choice experiment
Adult M. soledadinus individuals were offered different potential prey species to assess their dietary breadth. The prey species used were a mix of native and introduced invertebrates from the orders Coleoptera, Diptera, Lepidoptera and Haplotaxida (Table II). These species were chosen because they are abundant and widely distributed within the Kerguelen Archipelago, and they represent the major non-predatory macroinvertebrate groups present in the same habitats as M. soledadinus (i.e. fellfield, tundra and coastal). The offered prey also included juvenile M. soledadinus because predation of larvae by adults has been observed in the field. All individuals used in these experiments - prey and M. soledadinus - were manually collected along the coast from the research stations Port-aux-Français (70°12’59.76”E, 49°21’0.00”S) and Baie de l’Aurore Australe (70°11’10.50”E, 49°20’56.51”S).
Table II. Prey species offered to Merizodus soledadinus in feeding trials under different conditions (temperature, prey life stage and size, prey and predator abundance). Preys with abundances of 2 could be two of the same species or two different prey species.
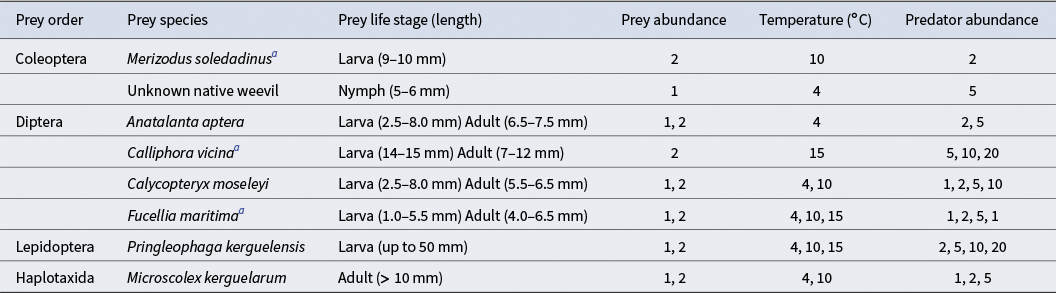
a Species not native to the Kerguelen Archipelago.
Trials were carried out in Petri dishes (diameter: 9 cm). The bottoms of the Petri dishes were covered with filter paper so that the insects were able to move more easily and to provide humid conditions through wetting of the paper with tap water. Predators fasted for 1 week prior to the start of the experiment. Prey individuals, alone or in combination with another of the same or different prey species, were placed in the Petri dishes prior to the introduction of the predators. We varied the number of M. soledadinus individuals to obtain different prey:predator ratios (1:1, 1:2, 1:5, 1:10 and 1:20) because this predator species is frequently found in high densities, especially under stones in fellfield areas at the Kerguelen Archipelago. In the Kerguelen Archipelago, the average monthly temperature ranges between 2°C in the winter and 8°C in the summer months (Frenot et al. Reference Frenot, Gloaguen, Massé and Lebouvier2001), although temperatures have been increasing recently (Daly et al. Reference Daly, Gerlich, Frenot, Høye, Holmstrup and Renault2023a). We conducted this experiment under two different temperature conditions typical of the daytime in the sub-Antarctic Kerguelen Archipelago during winter and summer periods (4°C and 10°C) and one appreciably above such temperatures (15°C) to capture a range of temperatures, allowing for the estimation of potential changes in invertebrate predator behaviour as a function of environmental conditions (Table II). Due to constraints on the availability of prey individuals and limits on collecting wild individuals from the local natural reserve, not all conditions could be tested for all species. For summaries of conditions by species and number of replicates by combination, see Tables II & III, respectively.
Table III. Proportion of prey consumed by Merizodus soledadinus in different prey choice feeding trials. Fractions show the number of prey consumed over the number of times each prey species was offered across trials with different predator abundances (1–20) in different temperatures (4°C, 10°C or 15°C).
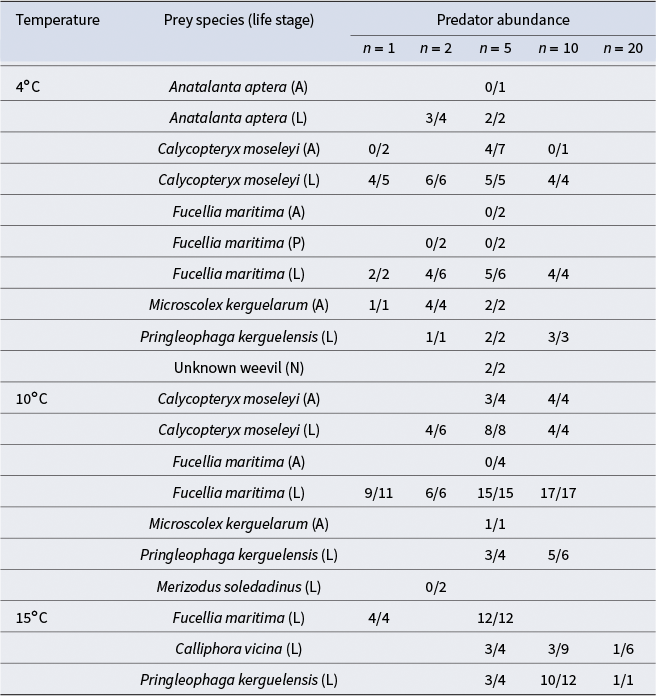
A = adult; L = larva; N = nymph; P = pupa.
M. soledadinus individuals were presented with either one or two prey individuals and were left with the prey for 24 h, after which we recorded the status of the prey as intact, partially consumed or totally consumed. Prey partially eaten but still alive within the observation period were considered partially consumed. We binary transformed our outcome (intact, partially consumed or totally consumed), with prey described as either consumed or intact. We used binary logistic regression to model predation by M. soledadinus during the first hour of the trial and at the end of 24 h with prey taxonomy, temperature (4°C, 10°C or 15°C), prey type (species), origin (i.e. native or non-native species), life stage (juveniles (larvae, nymph), pupae or adult) and predator density for the consumption of prey. Also included in the model was whether or not alternative prey were offered. We fitted several models using the ‘drop1’ function in the base R package stats (R Core Team 2023) and compared these to a simple model based on the life history traits of M. soledadinus in order to better understand what influences predatory behaviour in this species.
Y-tube olfactometer bioassays
Behavioural responses of adult M. soledadinus to prey or plant odours were examined by conducting olfactometric tests. A Y-tube olfactometer that had two glass arms of 20 cm each in length, a stem of 12 cm and an inner diameter of 1 cm (Analytical Research Systems, Inc., Gainesville, FL, USA) was used. A two-channel air delivery system (Analytical Research Systems, Inc.) was used for filtering and humidifying the incoming air drawn into the two arms of the Y-olfactometer at a constant rate of 1.5 ml/min. The olfactometer was placed horizontally under a 60 W red lightbulb placed 70 cm above the experimental setup. The Y-tube experiment was realized at 8°C ± 1°C and 70–80% relative humidity.
Scents were loaded from 1) one plant species, Acaena magellanica (Lam.) Vahl, 1771, and 2) decaying seaweed, representing terrestrial and coastal habitats, respectively. We also repeated this experiment with odours from potential prey species: the native fly A. aptera and the native caterpillar P. kerguelensis, which were either intact or injured. Lastly, we conducted another set of trials with starved and fed M. soledadinus. All bioassays were performed by presenting one stimulus vs one unbaited arm to individuals of M. soledadinus whose sex was not known. Bioassays were conducted by presenting the stimuli to 12 adult individuals (stimuli were placed six times in each arm of the Y-olfactometer) to M. soledadinus individuals used only once for each experimental condition. At the start of each experiment, one adult of M. soledadinus was placed in the stem of the Y-tube and allowed to acclimatize for 15 min. Then, the samples were introduced and the behaviour of the adult was monitored for 30 min; the choices made by each insect between one of the two arms were noted. For each assay, a choice was recorded when the adult M. soledadinus passed the intersection into one of the two arms and remained more than 15 s in that arm. Insects that made no choice were also counted. After every run, the Y-tube and the chambers were cleaned with ethanol and rinsed with water, and the connection of each chamber to the arm was reversed to take into account any potential position bias. To determine whether M. soledadinus responded to odour cues with increased activity in scented tubes, we again used a Kruskal-Wallis test, as the data were not normally distributed (plant data: W = 0.768, P < 0.05; prey data: W = 0.881, P < 0.05).
Results
Cooperative feeding by Merizodus soledadinus
We found significant differences (P < 0.05) in the time to first attack between single M. soledadinus individuals and groups of 10 individuals for all species examined (Fig. 1). When lone M. soledadinus individuals were presented with the caterpillar P. kerguelensis, none of the individuals attacked the caterpillars within the initial hour of observation, in contrast to all other treatment groups (Fig. 1). The groups of 10 predators presented with a caterpillar had the longest average time to attack of the remaining groups: 33 min 30 s (Fig. 1). The time to attack for single predators was similar for C. moseleyi and M. kerguelarum, taking ~20 min (Fig. 1). There was a shorter time to attack in treatment groups with 10 predators compared with the sole predator for all species (Fig. 1). The shortest times to first attack were observed for the groups with 10 predators and one larval dipteran (C. moseleyi) and one native worm (M. kerguelarum), with means of 6 min 36 s and 4 min 16 s, respectively.
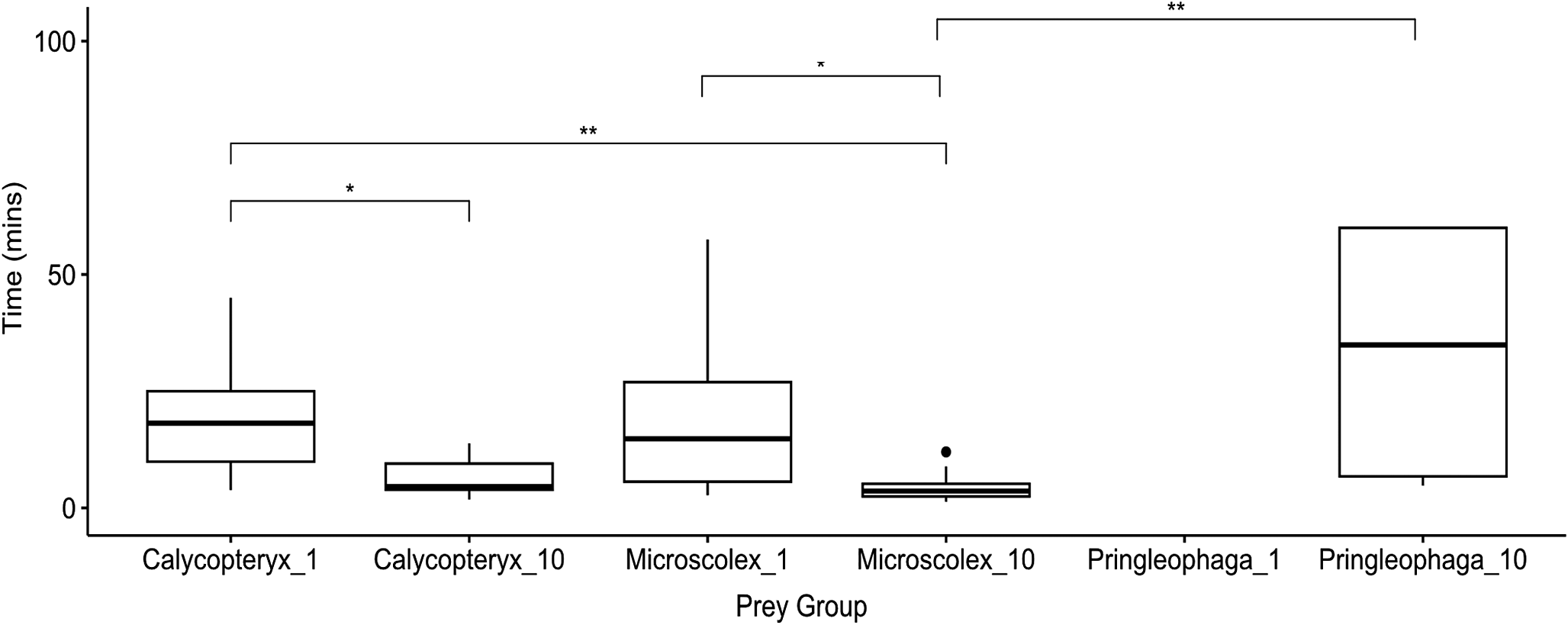
Figure 1. Boxplots showing time to attack by Merizodus soledadinus in experimental groups of three different prey species: Calycopteryx moseleyi, Microscolex kerguelarum and Pringleophaga kerguelensis. Trials were conducted with either 1 or 10 predators and always 1 prey individual. The number of replicates ranged from 8 to 22 and varied across condition due to prey availability (see Table I for details). Horizontal lines represent median time to attack, with upper and lower bounds of the boxes representing first and third quartiles. Maximum and minimum values are denoted by the whiskers, with dots denoting outlier values (data points 1.5 times smaller or larger than the interquartile range). Asterisks correspond to Bonferroni-adjusted significant differences between times to attack in different groups (* P < 0.05, ** P < 0.01) according to Dunn’s test.
In addition to differences in time to attack, there were also significant differences in mortality across prey type according to a two-tailed Fisher’s exact test (P < 0.05). After 5 h, all of the caterpillars remained alive and intact in the sole predator group, but 75% were dead in the 10 predator group. M. kerguelarum demonstrated 100% mortality in both treatment groups, whereas C. moseleyi demonstrated 94% and 100% mortality rates in the lone and group predator treatments, respectively.
Dietary breadth and prey preferences of Merizodus soledadinus
The best model was selected based on significance of predictors and contained only prey stage and species (model 7, Table S1). Prey life stage (z = 4.238, P < 0.05) was the only significant predictor of predation within 24 h. Prey being in juvenile stages (larva or nymph) increased the odds of consumption by M. soledadinus by 46 times relative to adult life stages (95% confidence interval = 46.3 ± 1.77). Conversely, prey in pupal stages showed decreased odds of consumption by M. soledadinus relative to adult life stages; however, this was not significant, and there were very few pupal replicates, and so conclusions cannot be drawn regarding predation on pupa. Some prey species approached significance as predictors of consumption by M. soledadinus; however, none of the individual species were significant predictors. C. moseleyi, Fucellia maritima, M. kerguelarum and weevils showed increased predation relative to A. aptera, whereas Calliphora vicina, M. soledadinus and P. kerguelensis showed decreased consumption (Fig. 2).
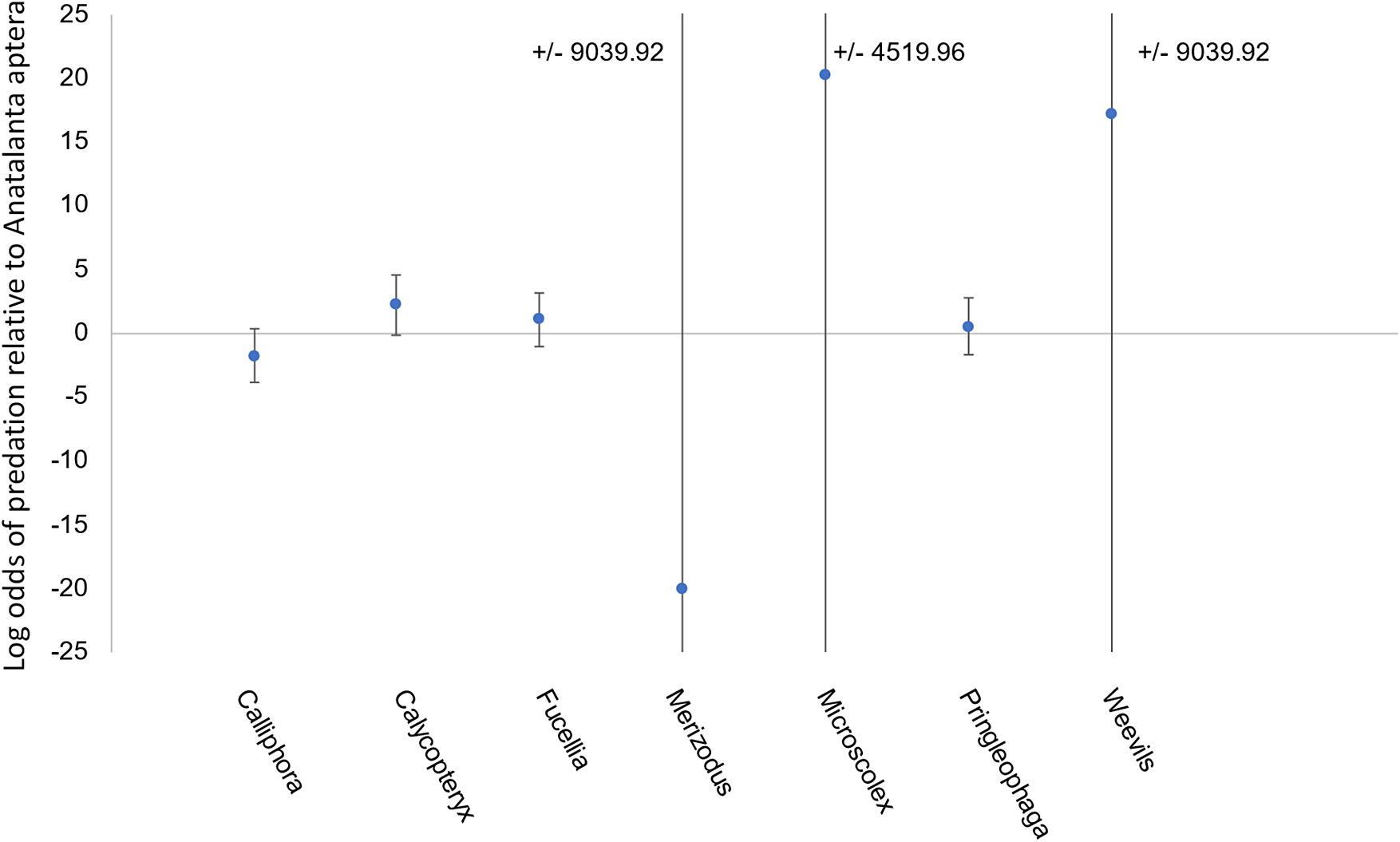
Figure 2. Model coefficients (average difference in log odds) for different species presented to Merizodus soledadinus in feeding trials. The odds of consuming Anatalanta aptera were used as the baseline in binary logistic regression, and points on this plot represent differences in consumption of other species relative to A. aptera from this regression. Plot margins are limited from -25 to 25, but error limits extending beyond this range are annotated on the plot. The number of replicates varied due to prey availability and can be found, along with the number of times each prey was consumed, in Table III.
The juvenile forms (larvae and nymphs) of the prey presented were generally readily consumed across species. The main exceptions were C. vicina larvae, which were not consumed in the majority of the cases, and M. soledadinus larvae, which were never consumed (Table III). F. maritima pupa, which were the only pupa tested, were also left intact, despite F. maritima larvae being readily consumed (Table III). Adult A. aptera and F. maritima were not consumed when offered, nor was C. moseleyi when there was a low number of predators; however, M. soledadinus did consume C. moseleyi adults when there were five predators present (Table III). Adult earthworms (M. kerguelarum) were consistently consumed in feeding trials (Table III).
Y-tube olfactometer bioassays
No significant differences were found in the time spent in the baited arm as compared with the fresh air sides for both A. magellanica (‘terrestrial’ stimuli) and seaweeds (‘marine’ stimuli; P > 0.05; Figs S1–S3). Similarly, no significant differences were found in the tube arms for the amount of time spent, distance travelled or number of visits between the terrestrial and marine stimuli trials (P > 0.05; Figs S1–S3). In trials with different prey (injured or intact), there were no significant differences in the amount of time, distance travelled or number of visits by M. soledadinus to loaded arms compared to arms containing fresh air (P > 0.05; Figs S4–S7).
Discussion
We modelled the choice of M. soledadinus to attack prey using binary logistic regression and found that predation was best predicted by prey species and life stage (Fig. 2 & Table S1). We observed that M. soledadinus is able and willing to attack a variety of prey from diverse taxonomic groups (i.e. Diptera, Lepidoptera, Coleoptera and Haplotaxida), but that it prefers juvenile forms (larvae and nymphs). Depending on life stage, we showed that M. soledadinus predated on all species tested except for its own (Table III), despite cannibalism being observed in this species in the wild. Nonsexual cannibalism has been relatively well studied in beetles and can occur due to high density, size differences and starvation (Scharf Reference Scharf2016). Larval cannibalism occurs in some carabids such as Pterostichus oblongopunctatus Fabricius, 1787 and Philonthus decorus Gravenhorst, 1802, even when other food is available (Heessen & Brunsting Reference Heessen and Brunsting1980), while in ladybirds this is constrained to occurring under conditions of low food quality or availability (Michaud Reference Michaud2003). It is possible that cannibalism in M. soledadinus is constrained to specific stress responses that we did not produce in our trials.
Although the Kerguelen Archipelago has low macroinvertebrate diversity, we could not test every macroinvertebrate species in our trials. However, given the generalist nature of M. soledadinus, as observed in our prey choice trials (Fig. 2 & Table III), we posit that this species may opportunistically predate upon any larval coleopterans, dipterans and lepidopterans available locally. These include the moth E. halticella Eaton, 1875, along with native flies and weevils (see Hullé & Vernon 2021). Similarly, native and introduced worms are also probably at risk of predation. The wide dietary breadth of M. soledadinus probably contributes to its invasive success (Romanuk et al. Reference Romanuk, Zhou, Brose, Berlow, Williams and Martinez2009) and may exacerbate its impacts. Invasive generalist predators can be particularly impactful in their novel ranges as they can drive prey populations to lower levels than native predators (Crowder & Snyder Reference Crowder and Snyder2010) and lead to top-down trophic cascades, where impacts propagate through multiple steps in a food web (David et al. Reference David, Thébault, Anneville, Duyck, Chapuis, Loeuille, Bohan, Dumbrell and Massol2017, Kehoe et al. Reference Kehoe, Frago and Sanders2021). In this scenario, theory predicts a ‘parity effect’ whereby species connected by an odd number of links to the invasive predator, such as its prey, are negatively affected and species connected by an even number of links to the invasive predator, such as the prey of its prey, are positively affected (Gallardo et al. Reference Gallardo, Clavero, Sánchez and Vilà2016, David et al. Reference David, Thébault, Anneville, Duyck, Chapuis, Loeuille, Bohan, Dumbrell and Massol2017). The impact of M. soledadinus may also be exacerbated due to the low species richness occurring at high trophic levels (Chown & Convey Reference Chown and Convey2016), resulting in low competition and enemy release in its invasive range.
Despite our results indicating that the majority of native macroinvertebrates present in the Kerguelen Archipelago are theoretically suitable prey for M. soledadinus, not all of these species may match well in terms of habitat or phenology. A more in-depth analysis should be performed to identify species most at risk, especially endemic and rare species. Care should also be taken when extrapolating the results from laboratory tests of prey choice because field conditions can also significantly influence prey choice. For example, McKemey et al. (Reference McKemey, Symondson and Glen2003) showed that although the predatory carabid P. melanarius preferred smaller prey in experimental trials, they fed more often on larger prey in field trials, probably because smaller individuals were better able to find refuge in a heterogeneous field environment. Prey of different sizes and from different taxonomic orders probably experience differential success in evading M. soledadinus in a field setting. M. soledadinus may also have indirect impacts on native predators, such as the spiders M. kerguelensis kerguelensis and N. antarcticus, as invasive insect generalist predators can have asymmetrical impacts on other predators through a number of mechanisms (Crowder & Snyder Reference Crowder and Snyder2010).
As insect life stage is an important determinant of predation by M. soledadinus, its impact probably depends on matching phenology, as demonstrated in other invasive insects (Russell & Louda Reference Russell and Louda2004). For example, if one species reproduces such that there are many larvae available during M. soledadinus’ activity or population peaks, there may be a much higher impact on that prey species than other similar prey, as has been demonstrated for the wingless Diptera A. aptera and C. moseleyi (Lebouvier et al. Reference Lebouvier, Lambret, Garnier, Convey, Frenot, Vernon and Renault2020). Phenological comparison of these two species should be carried out to determine whether this can explain the disproportionate impacts on A. aptera compared to similar native species. In the variable predator density and prey choice experiments, we showed that cooperative feeding between M. soledadinus individuals can improve predation success with difficult prey such as P. kerguelensis (Fig. 1 & Table S3), which could also have implications for prey choice depending on phenology and abundance along the invasion gradient. As this species expands its range, the prey it is able to successfully attack at the low population density range front may differ from that observed in established areas. In experimental trials with different densities of M. soledadinus predators and F. maritima prey, Géron et al. (Reference Géron, Cuthbert, Hotte and Renault2023) demonstrated that the proportion of attacked prey was positively related to predator density and negatively related to prey density, with a significant increase in attacks when five or more M. soledadinus individuals were present.
The lack of coevolutionary history between invasive and native species often leads to asymmetrical interactions benefitting invaders, as in the case of albatrosses and invasive mice on islands (Connan et al. Reference Connan, Jones, Risi, Smyth, Oppel and Perold2024), and sometimes this can even lead to extinction (Fritts & Rodda Reference Fritts and Rodda1998). These imbalances (see Daly et al. Reference Daly, Chabrerie, Massol, Facon, Hess and Tasiemski2023b for related hypotheses) can also create selective pressure on native species (David et al. Reference David, Thébault, Anneville, Duyck, Chapuis, Loeuille, Bohan, Dumbrell and Massol2017), as has been seen in the Italian agile frog’s (Rana latastei Boulenger, 1879) homogenization over diverse landscapes following invasion by a crayfish predator (Melotto et al. Reference Melotto, Manenti and Ficetola2020). As the main native macroinvertebrate predators in the Kerguelen Archipelago are spiders, native prey may be evolutionarily unprepared to resist predation by a predatory beetle with different feeding habits, including a preference for low-mobility larvae. However, little is known about prey life stage preferences and prey acquisition in native predators, making this hypothesis difficult to evaluate (Hullé & Vernon Reference Hullé and Vernon2021a,Reference Hullé and Vernonb). Whether this potentially functionally novel predator will cause enough pressure to lead to extinction of or selection in native species will depend on the adaptive capacity and life history traits of predator and prey, in addition to species-specific impacts of environmental change. Environmental change is likely to exacerbate these impacts, as warming in polar regions can favour invasive species relative to native ones, as with M. soledadinus, which is more tolerant to heat than many native sub-Antarctic species (Renault et al. Reference Renault, Leclerc, Colleu, Boutet, Hotte and Colinet2022).
Despite the differential thermal stress tolerance of M. soledadinus relative to native prey (Renault et al. Reference Renault, Leclerc, Colleu, Boutet, Hotte and Colinet2022), we found that the temperature at which the feeding trials were conducted was not included in the best predation models (Table S1). This was somewhat surprising, as thermal conditions are known to be important predictors of predator behaviour in ectothermic animals (Abram et al. Reference Abram, Boivin, Moiroux and Brodeur2017). This may be explained by the long timescale of observation (24 h), by the low number of replicates in different temperature regimes or potentially by the pre-adaptation hypothesis. This hypothesis states that successful invaders may be pre-adapted to conditions in their novel range because of conditions in their native range (Mack Reference Mack2003, Daly et al. Reference Daly, Chabrerie, Massol, Facon, Hess and Tasiemski2023b). As M. soledadinus is a native of the Falkland Islands and Patagonia (Johns Reference Johns1974), which have similar temperature regimes to the Kerguelen Archipelago (Lebouvier et al. Reference Lebouvier, Laparie, Hullé, Marais, Cozic and Lalouette2011), it may be pre-adapted to hunting in the low-temperature conditions of the Kerguelen Archipelago. Although the thermal regime of the Kerguelen Archipelago currently matches well with the native range and thermal requirements of M. soledadinus (Laparie & Renault Reference Laparie and Renault2016), climate change is rapidly increasing local temperatures (Lebouvier et al. Reference Lebouvier, Laparie, Hullé, Marais, Cozic and Lalouette2011), which could eventually favour invaders from more moderate climates. This is especially true if conditions become drier, as M. soledadinus is sensitive to desiccation (Ouisse et al. Reference Ouisse, Laparie, Lebouvier and Renault2017) and is thought to maintain a nocturnal lifestyle in the sub-Antarctic for this reason (Ottesen Reference Ottesen1990).
The Y-tube olfactometer experiment showed no significant preference of M. soledadinus for apparatus arms loaded with stimuli from different environments (i.e. terrestrial and marine), nor for different prey species. This may suggest that M. soledadinus is an opportunist, as it is not strongly attracted to any of the specific odours tested, or that M. soledadinus relies on other methods to find food, as predatory ground beetles can rely on multiple sensory inputs to detect prey. Experimental tests of predatory and omnivorous ground beetles have demonstrated species-specific responses to odours in the detection of food resources (Kielty et al. Reference Kielty, Allen-Williams, Underwood and Eastwood1996, Mundy et al. Reference Mundy, Allen‐Williams, Underwood and Warrington2000, Ali et al. Reference Ali, Mori, Prager and Willenborg2022), which we did not demonstrate in M. soledadinus. This species may instead rely on gustatory or tactile cues, as demonstrated in other Carabidae and Staphylinidae (Wheater Reference Wheater1989). Further testing is required to better understand the strategies used by this predator to detect prey.
Insect declines due to invasion are poorly studied compared to other major threats such as habitat loss, pollution, climate change and overexploitation (Sánchez-Bayo & Wyckhuys Reference Sánchez-Bayo and Wyckhuys2019), but the impacts of predation by introduced species can create transformative changes in invaded ecosystems (David et al. Reference David, Thébault, Anneville, Duyck, Chapuis, Loeuille, Bohan, Dumbrell and Massol2017). Despite this, due to the potential for adverse consequences for native species there are currently no control methods for established invasive macroinvertebrates in the Kerguelen Archipelago and sub-Antarctic (Jones et al. Reference Jones, Chown, Ryan, Gremmen and Gaston2003, Frenot et al. Reference Frenot, Chown, Whinam, Selkirk, Convey, Skotnicki and Bergstrom2005, Lebouvier & Frenot Reference Lebouvier and Frenot2007). The impacts of invasive generalist predators such as M. soledadinus and the limited management options for this species emphasize the need for strict biosecurity protocols to reduce further human-assisted dispersal of this species, as well as future introductions of non-native species. This is especially important in the context of global climate change, which is disproportionately affecting polar regions and could pave the way for the establishment of globally invasive species of concern in the Antarctic and sub-Antarctic (Duffy et al. Reference Duffy, Coetzee, Latombe, Akerman, McGeoch and Chown2017). Strict biosecurity controls and reduced access to the islands (relative to past farming and whaling eras) have successfully lowered the number of introduced species locally (Project 136-SUBANTECO from the French Polar Institute and long-term monitoring of the biota SEE-Life CNRS ‘Ecologie & Environment’). However, this vigilance must be maintained as long as there is a human presence on these islands, as well as there being monitoring efforts to catch species that have already been introduced but have not yet had the opportunity to establish or spread widely.
Supplementary material
To view supplementary material for this article, please visit http://doi.org/10.1017/S0954102025000082.
Acknowledgements
The authors thank E. Fayel and M. Laparie for technical assistance when performing the experiments.
Financial support
The authors were funded by the French Polar Institute Paul-Emile Victor (Project 136-SUBANTECO), CNRS-Ecology & Environment (Zone Atelier Antarctique et Terres Australes) and ANR (ANR-20-EBI5-0004, BiodivERsA, BiodivClim call 2019–2020, ASICS project).
Competing interests
The authors declare none.
Author contributions
DR designed the project and oversaw the experiments. ED performed the data analyses. ED and DR both contributed to the writing and editing of the manuscript.
Data accessibility statement
All data discussed in this study have been made freely available on GitHub at the following link: https://github.com/davidrenault/Trophic_ecology_Merizodus_soledadinus.








