Introduction
Leopard seals (Hydrurga leptonyx Blainville Reference Blainville1820) tend to live solitary lives, mainly associated with sea ice (Southwell et al. Reference Southwell, Kerry, Ensor, Woehler and Rogers2003). They have a circumpolar distribution, with the highest densities on the Antarctic Peninsula (AP; Rounsevell & Eberhard Reference Rounsevell and Eberhard1980, Borsa Reference Borsa1990, Rogers Reference Rogers, Perrin, Wursig and Thewissen2009). During the summer, which includes the breeding and moulting periods, leopard seals, as one of the four Antarctic pack-ice seal species (Erickson & Hanson Reference Erickson, Hanson, Kerry and Hempel1990, van der Linde et al. Reference van der Linde, Visser, Bout, Krause, Forcada and Siniff2022), occur throughout the pack ice that rings Antarctica, being most abundant within 300 m of the pack-ice edge (e.g. associated with polynyas; Condy Reference Condy1977, Bester et al. Reference Bester, Ferguson and Jonker2002). During years when the pack ice is most extensive, reaching farther north than average, vagrants appear at peri-Antarctic islands, such as Macquarie Island, as well as at New Zealand and Patagonia (Testa et al. Reference Testa, Oehlert, Ainley, Bengtson, Siniff, Laws and Rounsevell1991); they have shown up frequently enough in Patagonia (which is relatively close to the pack-ice edge, being just a few hundred kilometres distant) that breeding populations have been established in glacial fjords (van der Linde et al. Reference van der Linde, Visser, Bout, Krause, Forcada and Siniff2022, Borras-Chavez et al. Reference Borras-Chavez, Soteres, Gómez, Martínez, Fernández-Ferrada and Castillo-Aguilar2024).
The louse Antarctophthirus ogmorhini (Anoplura: Echinophthiriidae) parasitizes this seal, although little is known about its life cycle. Among Anopluran lice, the family Echinophthiriidae consists of species that primarily infest pinnipeds. Throughout their evolution, these lice have adapted to the semiaquatic lifestyle of their hosts by developing specialized morphological, ecological and behavioural traits (Kim Reference Kim1975, Leonardi et al. Reference Leonardi, Crespo, Soto and Lazzari2021). Pinnipeds are highly adapted to the aquatic environment but must regularly haul out on land or ice to rest, breed, pup or moult (Ray Reference Ray1963, Hindell et al. Reference Hindell, Bradshaw, Sumner, Michael and Burton2003, Kuhn & Frey Reference Kuhn and Frey2012), although the proportion of time spent ashore vs in the water varies by species (Bengtson et al. Reference Bengtson, Laake, Boveng, Cameron, Bradley Hanson and Stewart2011). This periodic haul-out behaviour is crucial for lice transmission, as their entire life cycle depends on direct host contact. One of the greatest challenges for these parasites is completing their development within a limited time frame, as seal lice cannot survive underwater for extended periods.
Consequently, lice reproduction and transmission are restricted to a host’s reproductive or moulting seasons, when they remain hauled out for longer durations (Aznar et al. Reference Aznar, Leonardi, Vera, Vales, Ameghino, Raga and Crespo2009, Leonardi et al. Reference Leonardi, Poljak, Carlini, Galliari, Bobinac and Santos2014). Previous studies on these lice have led to taxonomic confusion. Initially, A. ogmorhini was misidentified as the louse species parasitizing Weddell seals (Leptonychotes weddellii; Mehlhorn et al. Reference Mehlhorn, Mehlhorn and Plötz2002). Later, Leonardi et al. (Reference Leonardi, Poljak, Carlini, Galliari, Bobinac and Santos2014) clarified that A. ogmorhini is specific to leopard seals, whereas Antarctophthirus carlinii parasitizes Weddell seals. Despite their distinct host associations, Antarctophthirus lobodontis, A. carlinii and A. ogmorhini exhibit minimal genetic divergence, highlighting their evolutionary proximity while maintaining strict host specificity (Durden & Musser Reference Durden and Musser1994, Leonardi et al. Reference Leonardi, Virrueta Herrera, Sweet, Negrete and Johnson2019).
The immune systems of animals can be altered by various factors and environmental conditions (Davis et al. Reference Davis, Maney and Maerz2008), such as the presence of pathogens or parasites (Mos et al. Reference Mos, Morsey, Jeffries, Yunker, Raverty, De Guise and Ross2006), changes in body condition (Castellini et al. Reference Castellini, Davis, Loughlin and Williams1993, Brock et al. Reference Brock, Hall, Goodman, Cruz and Acevedo-Whitehouse2013), geography (Cammen et al. Reference Cammen, Hoffman, Knapp, Harwood and Amos2011) or breeding status (Deerenberg et al. Reference Deerenberg, Arpanius, Daan and Bos1997) or contaminants (de Swart et al. Reference de Swart, Ross, Vos and Osterhaus1996). Studies on leopard seal health assessment suggest that leopard seals may be sensitive to changes imposed upon them by their environment as well as the presence of lice (Gray et al. Reference Gray, Rogers, Canfield, Kerry and Riddle2009, Leonardi et al. Reference Leonardi, Virrueta Herrera, Sweet, Negrete and Johnson2019). This study aimed to analyse, for the first time, the host-parasite association between the leopard seal and A. ogmorhini. The infestation parameters of A. ogmorhini in leopard seals and the relationship between host body condition (see later) and the presence of lice were investigated on the Danco Coast, AP.
Materials and methods
Sample collection
During four fieldwork seasons (December–February in 2014, 2015, 2019 and 2020), we collected samples from 50 leopard seals (12 females and 38 males) in Antarctic Specially Protected Area (ASPA) No. 134, AP (Fig. 1). Seals were chemically sedated, and we recorded life-history traits (sex, age) and morphometric data (mass, length). The standard length was measured as the straight-line distance from the snout to the tip of the tail. Lice were collected from hind flippers using a plastic comb (Fig. 2) and preserved in 96% ethanol, following standard methods (Thompson et al. Reference Thompson, Corpe and Reid1998, Leonardi Reference Leonardi2014). The hind flippers are the preferred site for lice, and the numbers collected there provide a reliable proxy of the total burden while reducing host manipulation time (Thompson et al. Reference Thompson, Corpe and Reid1998, Leonardi Reference Leonardi2014). Seal handling and immobilization were approved by the Dirección Nacional del Antártico (Argentina). Sex, weight and age class were recorded for each animal. Animals were chemically sedated using Tiletamine/Zolazepam 1:1 (1.3 mg/kg) according to the procedures described in Higgins et al. (Reference Higgins, Rogers, Irvine and Hall‐Aspland2002).
Figure 1. General view of Antarctica and Antarctic Specially Protected Area (ASPA) No. 134 in the northern sector of the Danco Coast, Antarctic Peninsula.

Figure 2. Leopard seal hauled out in a small region of pack ice during the moulting season in the northern sector of the Danco Coast, Antarctic Peninsula. a. Detail of an Antarctophthirus ogmorhini louse. b. Detail of lice collection using a plastic comb.
Infestation parameter estimation
Infestation parameters were estimated following Bush et al. (Reference Bush, Lafferty, Lotz and Shostak1997) and Rózsa et al. (Reference Rózsa, Reiczigel and Majoros2000). Prevalence is defined as the proportion of infested hosts in the population, mean abundance is the mean number of parasites per host (considering infested and non-infested hosts) and mean intensity is the mean number of parasites per host (considering only infested hosts). The 95% confidence interval was calculated using the free software Quantitative Parasitology v.3 (Reiczigel et al. Reference Reiczigel, Zakariás and Rózsa2005), and mean values of intensity were calculated using the bias-corrected and accelerated bootstrap method with 20 000 replications (Rózsa et al. Reference Rózsa, Reiczigel and Majoros2000).
Body condition
We calculated body condition expressed as ‘relative condition (Kr)’ (Table I), as proposed by Chabot & Stenson (Reference Chabot and Stenson2002), being:
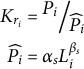
Table I. Individual information of each leopard seal (Hl).
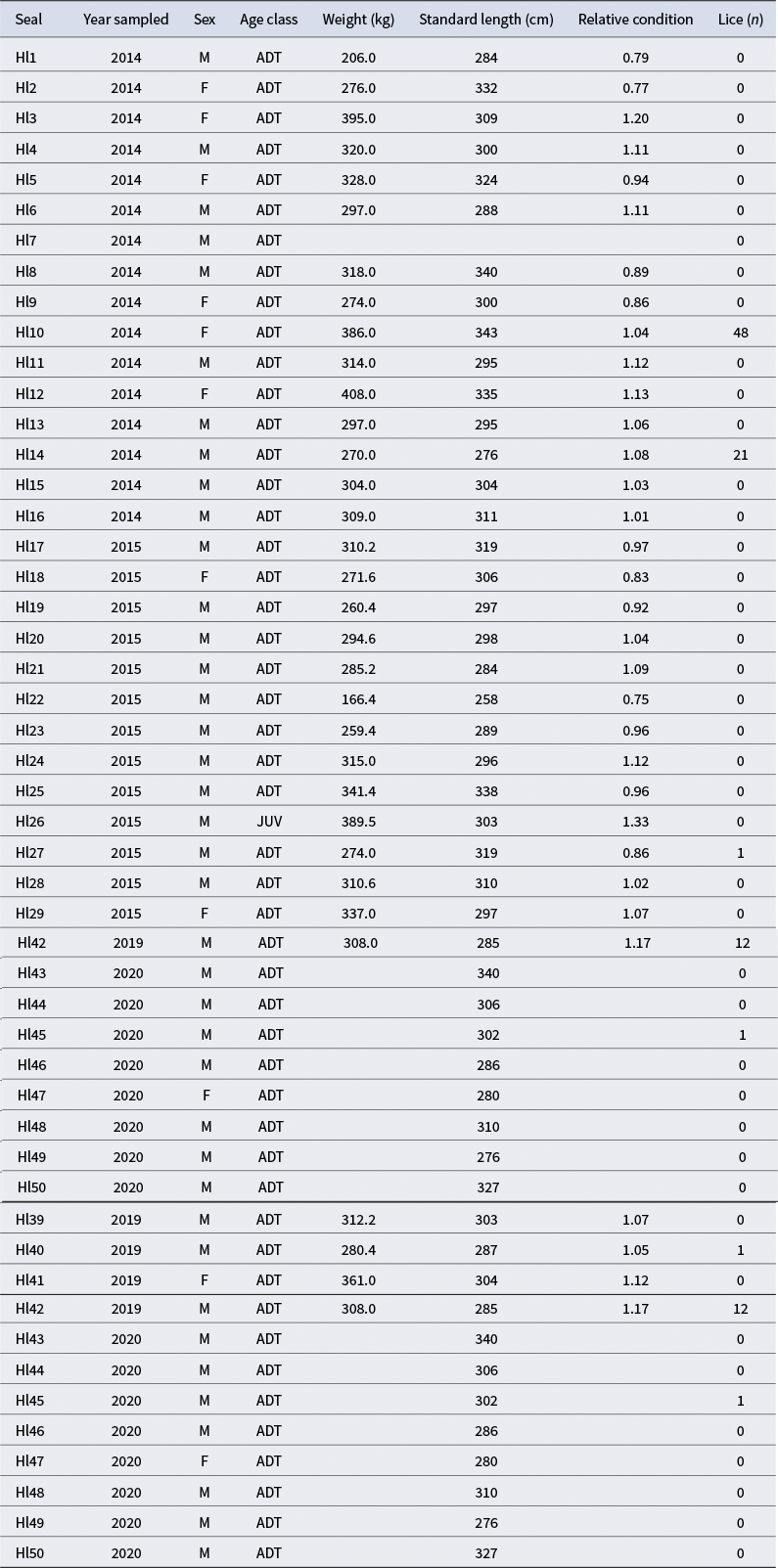
ADT = adult; F = female; JUV = juveniles; M = male.
where Pi
is the observed weight of the i-th seal,
![]() $\widehat{P_i}$
is the expected weight, derived from the exponential relationship between length and weight, Li
is the length of the i-th seal, s represents sex (we will adjust sex-specific length-weight relationships as some species have marked sexual dimorphism) and α and β are the sex-specific parameters of the exponential relationships between length and weight.
$\widehat{P_i}$
is the expected weight, derived from the exponential relationship between length and weight, Li
is the length of the i-th seal, s represents sex (we will adjust sex-specific length-weight relationships as some species have marked sexual dimorphism) and α and β are the sex-specific parameters of the exponential relationships between length and weight.
Then, if the seal has the average weight for its length, Kr = 1; if the seal has a weight greater than the average weight for its length, Kr > 1; and if the seal has a weight less than the average weight for its length, Kr < 1. In this way, species-specific body condition indices can be constructed, taking into account the sexual dimorphism and age class of the pinnipeds.
Models
We made generalized linear models (GLMs) with the prevalence and abundance of lice as a function of sex, age class, year of sampling and body condition. Since only one female and one juvenile had lice, we excluded the variables sex and age class from the analysis. We propose a new model that considers body condition and year of sampling by setting body condition as a fixed variable and year of sampling as a random variable. In this way, we analyse the effect of body condition on the response variable of interest while statistically controlling for interannual variability. In both cases, the variance explained by the random variable year of sampling was very close to zero, so we discarded it. This simplified the model to the following GLM:
Response variable ~ Body condition
Results
Individual information regarding each leopard seal (year, sex, age class, weight, standard length, relative condition and number of lice) is provided in Table I. The infestation parameters of A. ogmorhini collected from leopard seals from the Danco Coast are shown in Table II. Less than 7 in 50 leopard seals were infected with lice (prevalence (P) = 14%).
Table II. Infestation parameters (with 95% confidence intervals in parentheses) for Antarctophthirus ogmorhini from leopard seals by sex, age class and year of sampling.
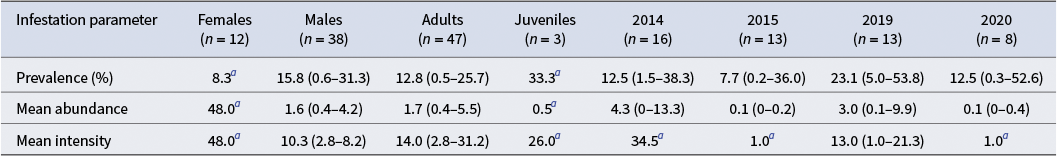
a There was only one infested individual.
Regarding the models, our data did not provide sufficient contrast to detect significant effects of sex or age class on lice prevalence or abundance. This effect of body condition on the 50% quantile of abundance was not significant (P = 0.43, slope = −498).
Discussion
Since the first description of A. ogmorhini (Enderlein Reference Enderlein1906) in leopard seals, there have been no further records or reports. Therefore, this study provides the first investigation of the infestation pattern of A. ogmorhini in this host. We sampled a total of 50 leopard seals, which is a huge sampling effort, not only because of to the solitary nature of these seals, but also because of their preference for resting on pack ice. These epidemiological studies provide important insights, particularly when studying a cryptic species living in the dynamic and rapidly changing environment of Antarctica.
The prevalence was the lowest in comparison to Lobodon carcinophaga (crabeater seal; P = 50%; Soto et al. Reference Soto, Klaich, Negrete and Leonardi2020), Leptonychotes weddellii (Weddell seal; P = 53.7%; Soto et al. Reference Soto, Negrete, Klaich and Leonardi2022) and Mirounga leonina (southern elephant seal; P = 62.5%; Ricca et al. Reference Ricca, Soto, Negrete and Leonardi2024). Furthermore, the prevalence was found to be lower in adults than in juveniles and lower in females than in males. The observed variation in prevalence among Antarctic seals may be influenced by their social behaviours. Whereas crabeater, Weddell and southern elephant seals are gregarious species, the leopard seal is predominantly solitary, typically hauling out on small patches of pack ice. It is an uncommon occurrence to observe more than one leopard seal on the same ice floe. The reproductive and transmission processes of lice are restricted to the host’s reproductive or moulting seasons and occur exclusively through direct contact between individuals (Murray & Nicholls Reference Murray and Nicholls1965, Kim Reference Kim1975, Leidenberger et al. Reference Leidenberger, Harding and Härkönen2007). Given the solitary nature of leopard seals, opportunities for A. ogmorhini transmission are limited, as direct contact among individuals is infrequent at least during the moulting season. This has been shown to reduce the chances of lice spreading, even when the hosts remain on ice for extended periods.
Furthermore, modelling analyses of three Antarctophthirus lice from leopard, crabeater and Weddell seals demonstrate that infestation parameters are contingent on the age class of the host (Soto et al. Reference Soto, Klaich, Negrete and Leonardi2020, Reference Soto, Negrete, Klaich and Leonardi2022). Consequently, juveniles exhibit higher levels of abundance and prevalence compared to adults. Specifically, a higher prevalence of lice was observed in juvenile leopard seals (P = 33.3%) compared to adult seals (P = 12.8%). However, only one of the three juveniles examined was found to have lice. Regarding sex, we found only one infested female, with 48 lice, while the mean intensity for males was 10.3. In order to detect significant effects of sex or age class on lice prevalence or abundance, it is necessary to increase the number of juveniles and females sampled. As females have been described as lice reservoirs in other species (Murray & Nicholls Reference Murray and Nicholls1965, Kim Reference Kim1975, Aznar et al. Reference Aznar, Leonardi, Vera, Vales, Ameghino, Raga and Crespo2009), it will also be interesting to sample leopard females during the reproductive season.
As previously stated, lice transmission is only possible when seals are on the sea ice (i.e. during mating, moulting or resting; Kim Reference Kim1975, Leidenberger et al. Reference Leidenberger, Harding and Härkönen2007, Leonardi et al. Reference Leonardi, Poljak, Carlini, Galliari, Bobinac and Santos2014). In this study, the presence of all stages of A. ogmorhini was observed, including eggs, nymphs and adults. This finding suggests that lice are capable of successful reproduction on their host during the summer, even during the moulting season. Lice may also reproduce during the seal-breeding season; however, whether this occurs remains unknown due to the lack of lice samples from that period for logistical reasons. The survival of eggs may be related to the diving and haul-out behaviour of the seals. In this regard, the reproductive strategies of A. ogmorhini lice appear similar to those of A. lobodontis on crabeater seals (Soto et al. Reference Soto, Crespo, Negrete and Leonardi2024), possibly due to the comparable diving behaviour of their hosts (Southwell et al. Reference Southwell, Bengston, Bester, Blix, Bornemann and Boveng2012). Leopard seals spend most of their time hauled out, followed by cruising and diving (Kuhn et al. Reference Kuhn, McDonald, Shaffer, Barnes, Crocker, Burns and Costa2006), with dives typically being shallow, generally to less than 100 m in depth (Southwell et al. Reference Southwell, Bengston, Bester, Blix, Bornemann and Boveng2012). This alternation of diving with short periods on ice is likely to allow lice transmission and reproduction. However, the relationship between seal dive behaviour and lice transmission has yet to be explored.
Our findings did not confirm a strong correlation between seal body condition and either the prevalence or mean abundance of lice. However, there is some evidence that seals in good body condition are more likely to be heavily infested (Table II). This is probably because in this parasite-host association that has co-evolved over the years, it is convenient and sufficient for the hosts to be in good body condition for the lice to feed and develop their life cycle properly. Following the body condition criteria proposed by Gray et al. (Reference Gray, Rogers, Canfield, Kerry and Riddle2009), we sampled seals in excellent, good and fair/thin body condition, most of which were males and adults. Thus, we cannot confirm the notion that lice are more prevalent in animals that are young, stressed or have underlying health issues (Wall & Shearer Reference Wall and Shearer1997).
In the future, collecting lice from tracked seals could be valuable to test hypotheses related to haul-out behaviour and diving patterns. This hypothesis proposes that seals that dive deeper and for longer durations, such as elephant seals and Weddell seals, primarily transmit adult lice. In contrast, seals that dive shallower and for shorter periods may allow lice to lay eggs and complete at least one generation. Additionally, conducting experimental pressure tests (Leonardi et al. Reference Leonardi, Crespo, Soto, Vera, Rua and Lazzari2020) and analysing the lice reproductive cycle could provide further insights into these strategies. Understanding host-parasite dynamics, particularly with respect to potential disease vectors such as blood-feeding insects, is crucial in the context of changing environmental conditions. Given that leopard seals have exhibited shifts in distribution, particularly in terms of their breeding areas moving northwards (Borras-Chavez et al. Reference Borras-Chavez, Soteres, Gómez, Martínez, Fernández-Ferrada and Castillo-Aguilar2024), it would be valuable to collect samples during the reproductive season from individuals giving birth in Patagonia to explore new hypotheses.
Acknowledgements
The authors support and defend the Argentinian Scientific Program, understanding science as an act of sovereignty. We thank Sebastián Poljak, Magalí Bobinac, Juan Galliari, Pedro Carlini, Julieta Cebuhar, Pablo Moscoso and Lucas Lanusse for their help with fieldwork and provision of lice samples and Paula Olivera for providing photographs. We also thank the reviewers for their comments.
Financial support
The study was financially and logistically supported by the Dirección Nacional del Antártico, Instituto Antártico Argentino. The permit for this work was granted by the Dirección Nacional del Antártico (Environmental Office). This research was funded by the Agencia de Promoción Científica Tecnológica (PICT 2015-0082, PICT 2018-0537) and PICTO Malvinas 2021-0021. This research was also funded by a Research Project from the Secretariat of Science and Technology of the UNPSJB (PI 1492- 80020180100018UP).
Competing interests
The authors declare none.
Author contributions
FAS, JN, AB and MSL conceived the idea for the study and designed the methodology; FAS, JN and MSL collected and processed the samples; FAS and AB analysed the samples and data; FAS and MSL led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.







