Introduction
Coronary artery bypass grafting (CABG) is the most common cardiac surgical procedure performed in the United States. Reference D’Agostino, Jacobs and Badhwar1 Surgical site infection (SSI) following CABG is a significant cause of morbidity and mortality. Reference Ahmed, Cheema and Ahmed2–Reference Greco, Shi and Michler4 Mitigating strategies include adherence to mandatory preoperative preventive requirements, including those established by the Surgical Care Improvement Project (SCIP) along with the Joint Commission. Despite these measures, approximately 1 to 4% of patients acquire infections after undergoing CABG. Reference Ahmed, Cheema and Ahmed2,Reference Gelijns, Moskowitz and Acker3,Reference De Lissovoy, Fraeman, Hutchins, Murphy, Song and Vaughn5–Reference Gummert, Barten and Hans7 The most common causative organisms in sternal wound infections are Staphylococcus species and gram-negative bacilli. Reference Sjögren, Malmsjö, Gustafsson and Ingemansson8 Deep SSIs, including mediastinitis, are associated with increased mortality rates. Reference Braxton, Marrin and McGrath9 Previous investigations of risk factors for SSI after CABG have yielded variable results. Reference Ahmed, Cheema and Ahmed2,Reference Zacharias and Habib10–Reference Slaughter, Olson, Lee and Ward12
Between the fall of 2018 and 2019, an increase in the occurrence of SSIs in patients who underwent a CABG procedure at our facility was noted despite standard SSI prevention measures, which included preoperative chlorhexidine skin preparation and intranasal povidone-iodine for all preoperative patients. Based on prior hospital surveillance data of SSI rates, these occurrences qualified a new cluster of SSIs and an investigation was initiated. The objectives of the investigation were to identify risk factors for SSI as well as implement measures to prevent additional cases.
Methods
This study was approved by our institutional research compliance office. The need for patient consent was waived given that the study was determined not to meet criteria for human subjects research through use of the VA Electronic Determination Aid (VAEDA).
Case definition and case ascertainment
A case was defined as any adult patient who acquired an SSI (superficial, deep, and organ/space) within 90 days of CABG surgery at this tertiary care medical center in Florida, USA during a 13-month period between the fall of 2018 and 2019 (study period). Case-patients were ascertained by review of the hospital’s infection control surveillance data. We compared the rates of all post-CABG SSIs during the pre- and post-cluster periods to identify the study period.
Case–control study
To identify patients at risk of acquiring an SSI, we compared the case-patient population with a randomly selected group of control-patients for a case to control ratio of 1:3 to ensure adequate power. The control population was defined as any adult patient who underwent a CABG surgical procedure during the study period but did not acquire an SSI. SSIs were classified as superficial, deep, or organ/space infections involving the primary CABG surgical site based on National Healthcare Safety Network (NHSN) criteria. Reference Horan, Andrus and Dudeck13,14 Variables analyzed included demographics, past medical history and co-morbidities, operating room procedures and timing, specific healthcare personnel involved in a patient’s care during surgery, vascular and airway access, surgical complications, duration of surgery, duration of medical device use, adherence to the SCIP measures and other perioperative standards of care (including surgical skin preparation, appropriate hair removal with clippers in the peri-operative area, appropriate timing and dosing/re-dosing of peri-operative antibiotics, and peri-operative maintenance of normothermia and normoglycemia), signs and symptoms of infection, exposure to and duration of cardiac bypass, enteral feeding, mechanical ventilation, urinary catheterization, types and duration of intravascular access including postsurgical intracardiac lines and devices, types and duration of intravenous antimicrobial therapy, receipt of blood products, and clinical outcomes.
Procedural review
Upon identification of the cluster in the second month of the study period, infection preventionists immediately conducted audits using standardized checklists to assess adherence to infection control practices and procedures in the operating rooms, hand hygiene, and environmental services cleaning protocols, including the cleaning of OR equipment. Of note, environmental services was adequately staffed and no changes were made regarding the type of cleaning products used or the frequency/location of cleaning. Any lapses found during audits were conveyed immediately to involved staff, and the OR manager and nursing cardiothoracic surgery leader subsequently monitored and enforced ongoing compliance.
Given ongoing cases despite the above measures, infection prevention personnel subsequently sought additional opinions and feedback from OR staff. These informal conversations occurred in group and individual settings. Staff raised concerns about operating room traffic during CABG procedures and infection preventionists directly observed the perfusion team removing their machinery when cardiac bypass was complete, resulting in prolonged door opening. As a result, policy was changed so that all equipment was left in place until the chest was closed. This change was implemented in the 11th month of the study period.
One surgeon also self-reported increased operation durations in part due to teaching activities and trainee involvement. As a result, additional limitations were placed on the time allotted to trainees during procedures. The cardiothoracic surgery team also reported that prior to the study period, only infected wounds healing by secondary intention received a vacuum-assisted closure device (wound VAC). Around the time of the study period, however, the VAC manufacturer provided new data supporting use in patients at higher risk of infection or dehiscence, leading to expanded use at our institution. In the 13th month of the study period, VAC use was phased out in favor of alternative methods, including surgical glue and VAC systems compatible with surgical glue dressings.
See Figure 1 for a graphical representation of the case distribution within the cluster as well as timing of the above interventions.
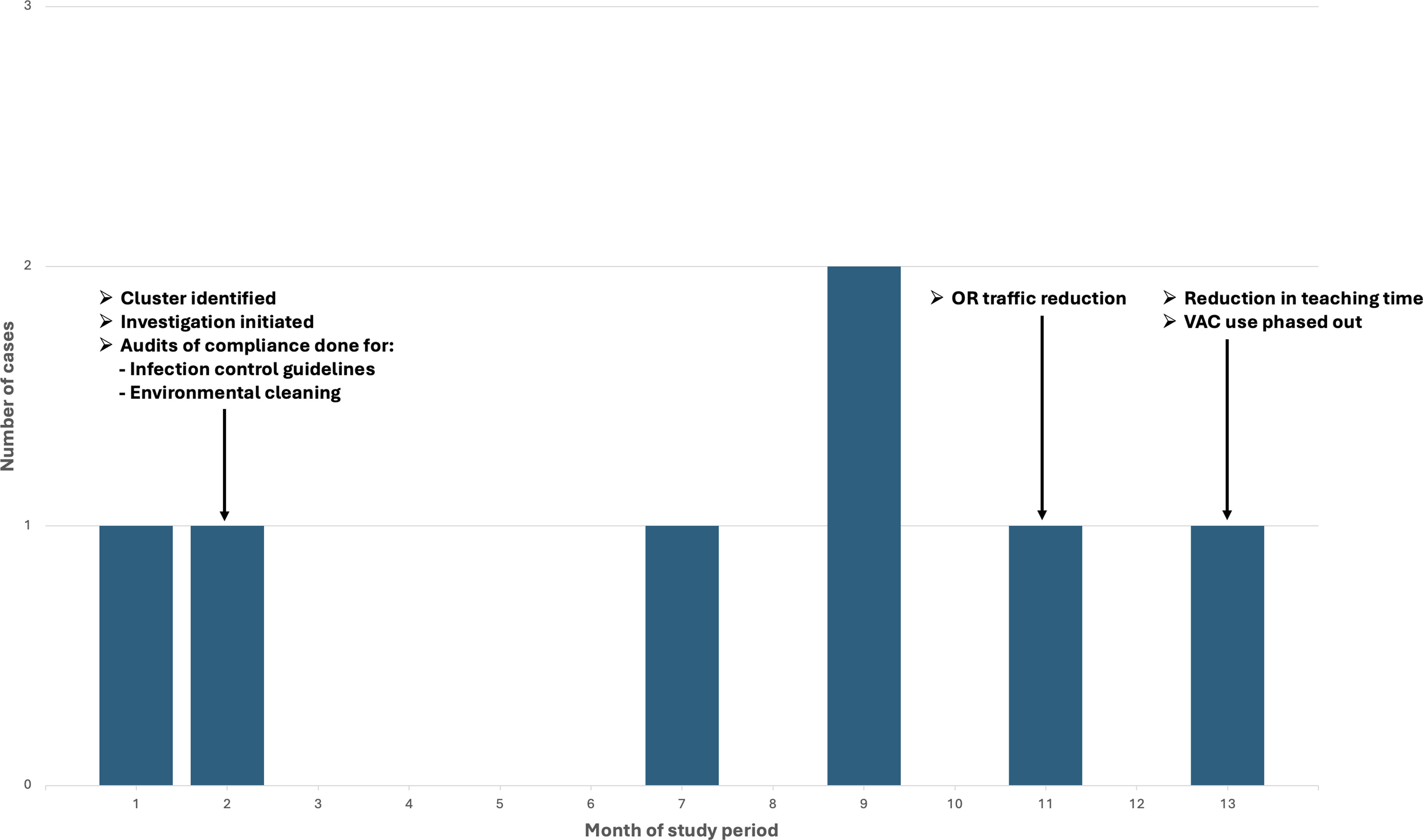
Figure 1. Distribution of cases during the study period including the timing of associated interventions.
Statistical analysis
All data were recorded on standardized forms, entered electronically, and analyzed using SAS statistical software Version 9.4 (SAS Institute Inc, Cary, North Carolina). Categorical variables were compared using the likelihood ratio test or, where appropriate, Fisher’s Exact test. Medians of continuous variables were compared using the Wilcoxon two-sample test. Odds ratios (OR) and 95% confidence intervals (C95) were calculated. P < 0.05 was considered statistically significant. We performed multivariate analyses and logistic regression to control for confounding variables and effect modification and to identify independent risk factors among those found to be statistically significant on univariate analysis.
Results
Case ascertainment and characteristics
During the study period, 118 total CABG surgeries were performed by two surgeons (58 by Surgeon A and 60 by Surgeon B). Seven adult patients met the case definition. Twenty-one control patients were randomly selected. All seven patients who met the case definition were male; six were Caucasian and one was Black. All of the control patients were Caucasian males. The median age of case-patients was 56 (range: 43–67) years; median body mass index (BMI) was 36 (range: 29–39) kg/m2. Of the seven patients meeting the case definition, two had superficial SSIs while five had deep SSIs per NHSN classification of surgical wound infections. Reference Horan, Andrus and Dudeck13,14 Two of the five patients with deep SSIs also had mediastinitis. Four patients had surgical cultures positive for methicillin-susceptible Staphylococcus aureus (MSSA), one patient had a culture positive for methicillin-resistant Staphylococcus aureus (MRSA), one patient had a culture positive for Escherichia coli, and surgical cultures were not obtained for one patient. Four patients had secondary bloodstream infections. Of note, molecular analysis was not performed on the MSSA isolates given that the associated surgeries were performed in months 1, 2, 8, and 9 of the study period and a common source of infection was felt to be unlikely. There were no patient deaths attributable to SSI, although one patient died from COVID-19 pneumonia over two years after his CABG surgical procedure.
Univariate and multivariate analysis of risk factors for SSIs after CABG
Case- and control-patients were similar regarding the presence of underlying cardiac disease, type of surgical procedure performed, exposure time on cardiac bypass, receipt of blood products, or type and duration of antimicrobials. On univariate analysis, case-patients were more likely than control-patients to have a significantly lower median age, higher median BMI, longer median duration of surgical procedure, placement of a wound VAC in the post-operative period, or to have successfully met one of the requirements of the SCIP (Table 1). On univariate analysis alone, case-patients were also statistically more likely to have had exposure to a particular surgeon (Surgeon A) and surgical fellow (Fellow X) during their surgery. All other variables analyzed were not statistically significant and are therefore not included in the table.
Table 1. Univariate and multivariate analyses of case-control study

* = significant in a multivariate analysis.
After controlling for confounding variables by performing multivariate analysis using logistic regression, independent risk factors for SSI among case-patients were lower age (P < 0.0001) and meeting the requirements of SCIP measure 10 (SCIP inf-10; perioperative temperature management to maintain normothermia [36.7 ± 0.6°C] with the exception of intentional hypothermia while on cardiac bypass) (P = 0.01). None of the other potential risk factors, including Surgeon A and Fellow X, were ultimately significant on multivariate analysis.
To further control for confounding, we directly compared the two surgeons who carried out the CABG procedures (Table 2). Univariate analysis revealed that patients operated on by surgeon A were more likely to have a longer duration of surgery (P = 0.0002), have spent a longer overall time in the operating room (P = 0.0002), have met SCIP measure 10 for maintaining perioperative normothermia (P = 0.01), have been on cardiopulmonary bypass longer (P = 0.02), and have HTN (P = 0.03) than patients operated on by surgeon B. There was a trend toward significance for younger patients as well (P = 0.05). Surgeon A was also more likely to have patients with a wound VAC in the post- operative period although the difference was not statistically significant. In contrast, Surgeon B was more likely to have patients with COPD (P = 0.01).
Table 2. Univariate and multivariate analyses comparing Surgeon A with Surgeon B
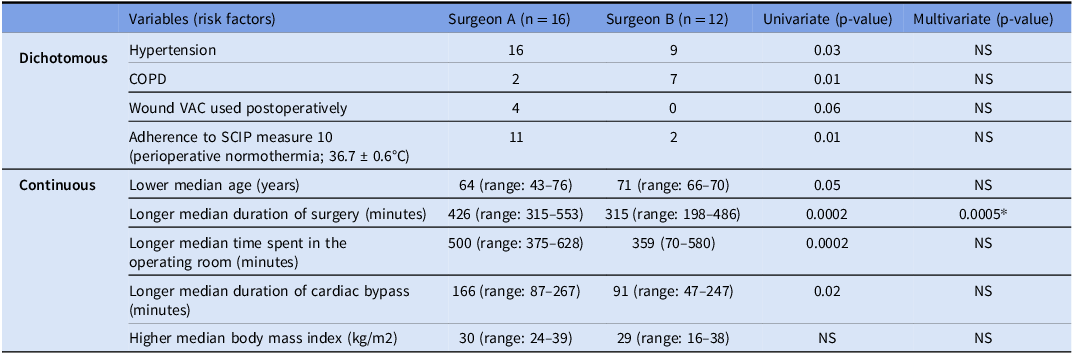
* = significant in a multivariate analysis.
Additional comparisons were made between the case-patients and non-case-patients for Surgeon A with regards to patient age, patient BMI, duration of bypass, and duration of surgery. Median duration of cardiac bypass and duration of surgery for case and non-case-patients in this comparison were similar. Patients who acquired SSIs were significantly more likely to be younger (P = 0.02) or have a higher BMI (P = 0.03), however (Table 3).
Table 3. Analysis of the 16 study patients who underwent surgery with Surgeon A

* = significant in analysis.
Procedural review results
As a result of the audits conducted by institutional infection preventionists, some lapses were identified with regards to hand hygiene (alcohol-based, not surgical scrub), and equipment cleaning (specifically tubing that had touched the floor). These lapses were corrected immediately in the second month of the study period and did not recur.
With regards to OR traffic reduction in the 11th month of the study period, the perfusion team immediately and permanently stopped removing equipment prior to chest closure. The interventions implemented in the 13th month of the study period, which included a reduction in trainee teaching time intra-operatively as well as phasing out VAC use, also became permanent changes.
After implementation of the above measures, no additional post-CABG cases were identified and SSI rates returned to baseline. In addition, no further SSI clusters were identified in the following three years.
Discussion
Previously identified risk factors for SSI following CABG include obesity, prolonged operative time, diabetes mellitus, hypertension, renal failure, and female gender. Reference Ahmed, Cheema and Ahmed2,Reference Pan, Tan, Cao and Feng15–Reference Meszaros, Fuehrer and Grogg17 Study results have been variable, however, in part due to institution-specific and local influences. Reference Ahmed, Cheema and Ahmed2 Optimizing infection control and antimicrobial stewardship practices can significantly improve infection rates. Reference Frenette, Sperlea, Tesolin, Patterson and Thirion18
Our results from this retrospective case-control study show that in this institution, lower age, increased BMI, longer duration of surgery, postoperative wound VAC use, and appropriate perioperative temperature management were univariate predictors of SSI risk, while only lower age and appropriate perioperative temperature management were independent predictors of infection in patients undergoing CABG surgery upon multivariate analysis. Surgical staff were not associated with an increased relative risk of infection on multivariate analysis after adjustment for covariates was performed.
Identified risk factors from our analyses and procedural review are discussed in more detail below.
Age
Increased age is a known risk factor for infection after cardiac surgery. In a study by Meszaros et al., for example, age greater than 74 years was the most significant risk factor for sternal infection after CABG. Reference Meszaros, Fuehrer and Grogg17 Functional status and comorbidities may potentially play a role in this association. Data for young adults is limited, but younger patients who develop premature coronary artery disease are more likely to have complex medical comorbidities, although overall mortality is typically less. Reference Dani, Minhas and Arshad19–Reference Biancari, Gudbjartsson and Heikkinen21 In our study, the median age of patients in the control group was 71 years whereas the median age for patients who developed SSIs was 56 years. The difference between these two groups was statistically significant as a risk predictor on multivariate analysis, indicating that younger patients were in fact associated with a higher risk for an SSI. Risk factors including elevated BMI, comorbidities such as HTN and COPD, and increased complexity of surgery requiring prolonged intraoperative time may have contributed.
Perioperative temperature management
Perioperative normothermia is a well-documented strategy to prevent complications including wound infections, coagulopathy, ischemic cardiovascular events, and mortality. Reference Sessler22–Reference Frank, Fleisher, Breslow, Olson and Beattie25 Since hypothermia results in vasoconstriction, decreased oxygen delivery to the surgical wound site may contribute to the increased risk of infection. Reference Scott, Stonemetz and Wasey23,Reference Sheffield, Sessler and Hopf26 SCIP inf-10, which is part of the evidence-based SCIP measures to improve perioperative care, outlines the acceptable range of perioperative temperatures. Reference Scott, Stonemetz and Wasey23 A number of studies, however, have reported poor correlation between SCIP compliance and patient outcomes, including SSIs. Reference Scott, Stonemetz and Wasey23,Reference Rasouli, Jaberi, Hozack, Parvizi and Rothman27–Reference Brown, Curry and Hyder30 . In our study, six out of seven patients who developed SSIs appropriately met the SCIP inf-10 measure for perioperative normothermia, and meeting this measure was also significant on multivariate analysis as a risk factor for SSI. Our findings are therefore consistent with prior studies suggesting a potentially poor correlation between SCIP measure compliance and reduction in SSIs. Additional limiting factors could include small sample size and variability in the location of temperature monitoring.
Body mass index
Obesity is a known risk factor for infection. Reference Chan, Sultan and Gleason31 Fat is more poorly vascularized, resulting in decreased tissue oxygenation and a higher risk of fat necrosis. Reference Thelwall, Harrington, Sheridan and Lamagni32,Reference Kabon, Nagele and Reddy33 Peri-operative antimicrobials are more likely to be underdosed in obese patients. Reference Ahmed, Cheema and Ahmed2 In addition, obesity itself may complicate surgical procedures and increase operative time. Reference Thelwall, Harrington, Sheridan and Lamagni32 Since higher median BMI was a statistically significant risk factor in this study on univariate analysis, although not on multivariate analysis, our findings are consistent with prior studies.
Duration of surgery
Prolonged surgical duration was significantly associated with increased risk of SSI in our univariate analysis, although not on multivariate analysis. CABG typically requires increased operative time given multiple surgical incisions and venous grafting. Wounds that are left open are more likely to become contaminated with skin flora or other bacteria in the operating room. Reference Pan, Tan, Cao and Feng15,Reference Cheng, Chen, Soleas, Ferko, Cameron and Hinoul34 In addition, tissue desiccation at the site of the incision may increase this risk. Reference Nguyen, Shugart and Lines29,Reference Haridas and Malangoni35 Operation duration may be associated with provider fatigue. Reference Nguyen, Shugart and Lines29 Prior studies have noted additional possible contributing factors, including intraoperative teaching. Reference Campbell, Henderson and Englesbe36 In this study, Surgeon A self-reported increased operation durations in part due to trainee involvement, as opposed to Surgeon B, who had less teaching time. This may have contributed to the longer average duration of surgery that was statistically significant on multivariate analysis when comparing Surgeon A and Surgeon B, although neither Surgeon A nor Fellow X were associated with an increased risk of SSI after CABG on multivariate analysis in the overall case-control study. Surgeon A subsequently placed additional limitations on the time allotted to trainees to decrease overall OR time.
Wound VAC use
Use of negative pressure wound therapy after cardiac surgery has previously been shown to be safe and effective. Reference Deniz, Gokaslan and Arslanoglu37,Reference Bapat, El-Muttardi, Young, Venn and Roxburgh38 Reported benefits of using a VAC system include increased blood flow and granulation tissue formation, decreased bacterial colonization, and a reduction in dressing changes. Reference Sjögren, Malmsjö, Gustafsson and Ingemansson8,Reference Bapat, El-Muttardi, Young, Venn and Roxburgh38 Complications of prolonged VAC therapy have been reported, however, and include unhealthy granulation tissue formation, nonunion, and osteomyelitis. Prolonged exposure to suction may contribute to tissue damage. Reference Bapat, El-Muttardi, Young, Venn and Roxburgh38 Our cardiothoracic surgery team had expanded VAC use around the time of the study period based on new manufacturer data. VACs were used primarily in patients with higher BMI’s and large open wounds, which may already have increased the risk of infection. The initial association between SSI and VAC use in univariate but not multivariate analysis may therefore be related to patient characteristics and surgical complexity, but tissue damage from prolonged VAC use remains a possibility. VAC use was subsequently phased out at our institution.
Operating room traffic
Our investigation raised concerns about operating room traffic during CABG surgeries, specifically in relation to perfusion team protocols. Studies have shown that door opening during surgery may disrupt airflow dynamics and increase colony forming units in the operating room. Reference Young and O’Regan39,Reference Scaltriti, Cencetti, Rovesti, Marchesi, Bargellini and Borella40 Associated distraction has also been linked to surgical mistakes. Reference Young and O’Regan39 As a result, our protocols were modified to reduce prolonged door opening prior to chest closure.
Limitations of this study include the relatively small number of cases in the cluster, which may have affected precise estimates of key predictor effects for important categorical predictors in the multivariate model, rendering model parameter estimations unstable. We found, however, that statistically significant differences ascertained on univariate analyses enabled generation of a plausible hypothesis to explain the underlying risk factors for infection. In addition, only male patients were present in the cluster and so gender could not be assessed as a risk factor. Finally, because this was a retrospective case-control study, we were not able to document long-term sequelae of infection in case-patients.
In conclusion, multiple risk factors can affect SSIs after CABG, although prior studies evaluating these factors have yielded varying results. While investigating a cluster of SSIs at our institution, we found multiple variables potentially associated with increased SSI risk on univariate analysis, and after performing multivariate analysis using logistic regression, we identified younger age and meeting SCIP measure 10 for maintenance of perioperative normothermia as statistically significant independent risk factors for SSI. After the implementation of measures directed toward minimizing operating room traffic and phasing out wound VAC use, no additional clusters of SSIs were observed.
Acknowledgements
All authors report no conflicts of interest or financial disclosures relevant to this article. Jennifer D. Connolly acknowledges supported time for this study through her DNP program at the University of Colorado.
This article is the result of an unfunded study supported with resources and through the use of facilities within the Veterans Health System. The contents of this article represent the views of the authors and not necessarily the views of the Veterans Health System or the United States Government.






