Autism spectrum disorder (ASD) is characterised by enduring impairments in reciprocal social communication, social interaction and the presence of restricted, repetitive patterns of behaviour, interests or activities. 1,Reference Lai, Lombardo and Baron-Cohen2 It represents a lifelong neurodevelopmental condition, with a global prevalence of 0.82% among children aged between 6 and 12 years, Reference Talantseva, Romanova, Shurdova, Dolgorukova, Sologub and Titova3 exhibiting a higher occurrence in males than females. Reference Loomes, Hull and Mandy4 ASD stands as one of the most debilitating developmental disorders and imposes a significant economic burden. Reference Leigh and Du5
The causes of ASD remain mostly unclear; however, existing evidence suggests it has a complex origin, involving a significant genetic element along with environmental, physical and psychological factors. Reference Hallmayer, Cleveland, Torres, Phillips, Cohen and Torigoe6–Reference Zhao and Nyholt11 Among these many risk factors, maternal depression is particularly noteworthy because it is relatively common, affecting 21% of women during pregnancy Reference Yin, Sun, Jiang, Xu, Gan and Zhang12 and 14% postnatally. Reference Liu, Wang and Wang13 Maternal depression may serve as an indicator of chronic psychological stress, capable of triggering inflammation during pregnancy. Reference Christian, Franco, Glaser and Iams14,Reference Kinney, Munir, Crowley and Miller15 This inflammatory response may have implications for the neurodevelopment of the unborn child. Reference Estes and McAllister16 For instance, antenatal depression has been correlated with elevated fetal cortisol levels, which contribute to alterations in fetal hypothalamic-pituitary-adrenal axis activity and brain function. Reference Talge, Neal and Glover17 Studies further suggest that antenatal depression may induce epigenetic dysregulation in the offspring’s hypothalamic-pituitary-adrenal pathways and serotonin transmission. Reference Nemoda and Szyf18 This dysregulation has the potential to result in heightened offspring hypothalamic-pituitary-adrenal reactivity and alterations in brain serotonin levels, ultimately influencing the neurodevelopment of the offspring and raising the risk of ASD. Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin19
The availability of evidence concerning the risk of ASD in offspring with mothers experiencing depression is crucial for timely and suitable interventions for at-risk children. However, there is a notable lack of consistency in the findings of existing epidemiological studies. Although some studies have demonstrated links between maternal pre- and perinatal depression and an elevated risk of ASD in offspring, Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Say, Karabekiroğlu, Babadağı and Yüce28 others found no increased risk. Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29–Reference Croen, Grether, Yoshida, Odouli and Hendrick31 For instance, although a large prospective cohort study conducted in Taiwan by Chen et al Reference Chen, Chen, Hsu, Huang, Bai and Chen21 identified more than a twofold increase in ASD risk among offspring of mothers with depression, a retrospective cohort study in the USA by Brennan et al Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29 found no association between maternal antenatal depression and ASD in offspring. Moreover, one systematic review and meta-analysis conducted 5 years ago reported the risk of developing ASD in offspring exposed to maternal depression, but its findings were based on three studies and were limited to antenatal exposure. Reference Ayano, Maravilla and Alati32 These discrepancies, coupled with the absence of updated evidence on the subject, underscore the necessity for comprehensive evidence to address conflicting findings within the current literature and provide up-to-date pooled estimates. Therefore, this systematic review and meta-analysis aims to fill these gaps by thoroughly investigating the relationship between maternal pre- and perinatal depression and the risk of ASD in children and adolescents. The findings from this review have significant potential to inform preventive strategies, clinical guidelines and public health initiatives targeting maternal mental health support, optimal neurodevelopment promotion and the reduction of ASD risk in offspring.
Method
Study design
The results of this systematic review and meta-analysis were reported in accordance with the guidelines outlined by the Preferred Reporting Items for Systematic Review and Meta-Analysis Statement (PRISMA). Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche and Ioannidis33 Additionally, the protocol for conducting this systematic review and meta-analysis was pre-registered in the International Prospective Register of Systematic Reviews and Meta-analysis (PROSPERO) under the registration number CRD42023420211 (see Supplementary Table 1).
Data sources and searches
We conducted a comprehensive search for relevant articles across six databases, including PubMed, Medline, EMBASE, Scopus, CINAHL and PsycINFO, covering the period from the inception of each database to 21 February 2024. The search employed appropriate subject headings and keywords related to ASD and maternal depression. These included (‘neurodevelopmental disorder’ OR ‘Autis*’ OR ‘autism spectrum disorder*’ OR ‘autistic disorder*’ OR ‘ASD’ or ‘Asperger syndrome’ OR ‘pervasive developmental disorder*’ OR ‘Rett’s syndrome’ OR ‘childhood disintegrative disorder*’) AND (‘Maternal depression’ OR ‘prenatal depression’ OR ‘postnatal depression’ OR ‘postpartum depression’ OR ‘antenatal depression’). In addition to the database searches, we reviewed the reference lists of included studies to identify any additional relevant studies. The search strategy was formulated with the assistance of a medical librarian, and a comprehensive list of all search terms can be accessed in the supplementary information (see Supplementary Table 2).
Eligibility criteria
We included all observational studies (case–control and cohort) that investigated maternal depression as the exposure variable, irrespective of the timing (before and during pregnancy, or after childbirth). These studies were required to assess ASD as an outcome in children and adolescents. Eligible studies reported relevant statistical measures, such as odds ratios, hazard ratios and relative risks, along with their associated 95% confidence intervals, or provided data allowing us to calculate these effect estimates. There were no restrictions on the publication year, and we considered studies published in English for inclusion. Conversely, animal studies, case reports, case series, correspondence, abstracts and reviews were excluded from consideration.
Definition outcome and exposure variables
The study considered ASD as the dependent variable, which is determined according to established diagnostic criteria such as the DSM Reference Cowan34 and the ICD. 35 Maternal depression, whether occurring before, during or after the pregnancy period, was treated as the independent variable and assessed by well-recognised tools for diagnosing or screening for depression, such as the ICD, DSM, Edinburgh Postnatal Depression Scale (EPDS) and the Center for Epidemiological Studies Depression Scale (CES-D).
Data extraction
We began by identifying relevant studies and importing them into our citation manager (Microsoft EndNote version 20 for Windows, Clarivate, Philadelphia, PA, USA; see https://endnote.com/downloads/), ensuring the removal of any duplicate entries. Subsequently, we used Rayyan software (Rayyan Systems, Cambridge, MA, USA; see https://www.rayyan.ai/) to conduct title and abstract screening. Two reviewers (B.T. and A.B.W.) independently evaluated titles, abstracts and full texts to identify articles that met our eligibility criteria. Any disagreements between the two reviewers were resolved through discussion.
These same two reviewers then independently extracted data from the included articles on 28–29 February 2024. The extraction process entailed recording details such as the first author’s name, year of publication, country of study, study design, sample size, types of maternal depression, instruments used for measuring exposures and outcomes, period of outcomes measured, confounders adjusted for, and measures of effect (including odds or hazard ratios, or relative risks) along with their respective 95% confidence intervals. In instances where studies provided multiple estimates, preference was given to those that underwent the most comprehensive adjustments. When studies presented various types of outcomes or measured a single outcome at different exposure times, each was treated as a distinct set of data.
Study quality appraisal
We utilised the Newcastle–Ottawa Scales (NOSs) to evaluate the methodological quality and risk of bias in studies. Reference Peterson, Welch, Losos and Tugwell36 These tools incorporated specific criteria, including the selection of study groups (4 points), comparison of groups (2 points) and ascertainment of the desired outcome (3 points). The scoring system for these tools ranged from 0 to 9, allowing the categorisation of studies into low-quality (1–4), medium-quality (5–7) and high-quality (8–9) groups. The assessment of study quality was independently conducted by B.T. and A.B.W., with any discrepancies resolved through discussion between them.
Data synthesis and statistical analysis
We conducted a meta-analysis using random-effect models to synthesis the available evidence. Our summary effect estimates were presented with the odds ratio accompanied by 95% confidence intervals. To assess statistical heterogeneity across the included studies, Cochran’s Q and the I 2-statistic test were utilised. Reference Cowan34,Reference Ioannidis37 Furthermore, subgroup analyses were conducted to examine differences in outcomes based on specific criteria, including time and measure of exposure, study design, year of publication, quality of study and some adjusted factors. To investigate the possibility of publication bias, we examined funnel plots and conducted Egger’s regression test. Reference Vandenbroucke38 All analyses were carried out with R software version 4.3.1 for Windows (The R Project, The R Foundation, Vienna, Austria; see https://cran.r-project.org/bin/windows/base).
Results
Search results
The search strategy and manual search identified 2459 records in the database search. After eliminating duplicates, a total of1987 records were screened based on their titles and abstracts for eligibility. Among these, 400 publications were found to be suitable for a full-text review. Finally, 12 studies were included in this systematic review and meta-analysis (Fig. 1). Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Croen, Grether, Yoshida, Odouli and Hendrick31
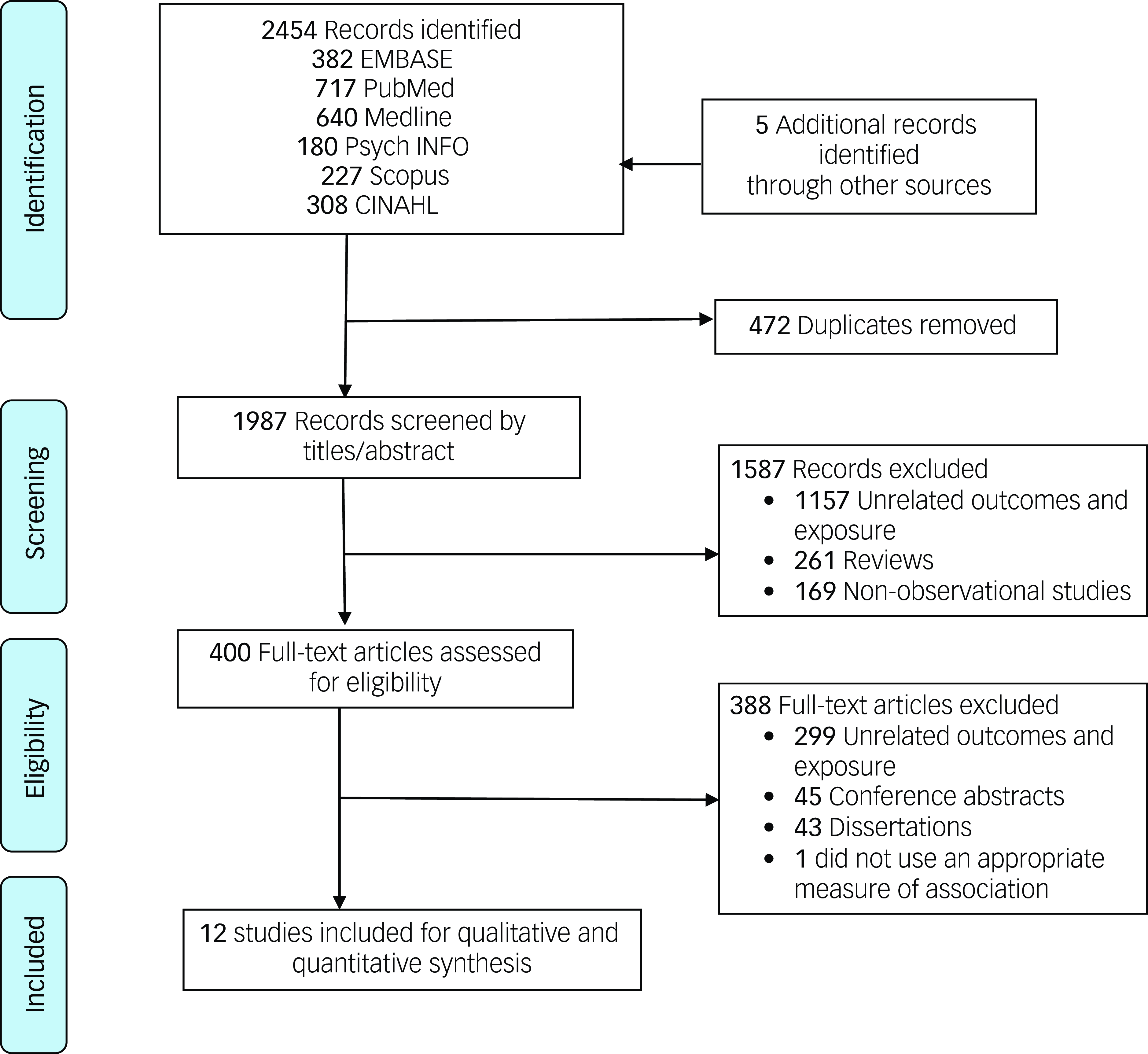
Fig. 1 The PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Review and Meta-113 Analysis.
Characteristics of included studies
Table 1 provides key characteristics of the studies included in this systematic review and meta-analysis. A total of 12 articles, comprising 1 655 966 mother–offspring pairs, were included. Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Croen, Grether, Yoshida, Odouli and Hendrick31 These studies were conducted from 2011 to 2023 across seven different countries. Five of the studies were conducted in the USA, Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20,Reference Clements, Castro, Blumenthal, Rosenfield, Murphy and Fava22,Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29–Reference Croen, Grether, Yoshida, Odouli and Hendrick31 two in Turkey Reference Güneş, Tanıdır, Doktur, Yılmaz, Yıldız and Özbek24,Reference Say, Karabekiroğlu, Babadağı and Yüce28 and another two in Denmark. Reference Gidaya, Lee, Burstyn, Yudell, Mortensen and Newschaffer23,Reference Hviid, Melbye and Pasternak26 Among the studies included in our analysis, eight were case–control studies Reference Clements, Castro, Blumenthal, Rosenfield, Murphy and Fava22–Reference Hagberg, Robijn and Jick25,Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27,Reference Say, Karabekiroğlu, Babadağı and Yüce28,Reference Castro, Kong, Clements, Brady, Kaimal and Doyle30,Reference Croen, Grether, Yoshida, Odouli and Hendrick31 and the remaining four were cohort studies. Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20,Reference Chen, Chen, Hsu, Huang, Bai and Chen21,Reference Hviid, Melbye and Pasternak26,Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29 All studies utilised diagnostic tools such as the ICD and DSM to assess ASD. Additionally, six studies employed these tools to evaluate maternal depression. Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Clements, Castro, Blumenthal, Rosenfield, Murphy and Fava22,Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27,Reference Castro, Kong, Clements, Brady, Kaimal and Doyle30,Reference Croen, Grether, Yoshida, Odouli and Hendrick31 Out of the 12 studies analysed, nine reported antenatal depression, Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20,Reference Chen, Chen, Hsu, Huang, Bai and Chen21,Reference Güneş, Tanıdır, Doktur, Yılmaz, Yıldız and Özbek24–Reference Castro, Kong, Clements, Brady, Kaimal and Doyle30 three focused on postnatal depression Reference Chen, Chen, Hsu, Huang, Bai and Chen21,Reference Güneş, Tanıdır, Doktur, Yılmaz, Yıldız and Özbek24,Reference Say, Karabekiroğlu, Babadağı and Yüce28 and five investigated pre-pregnancy depression as the exposure variable. Reference Chen, Chen, Hsu, Huang, Bai and Chen21–Reference Gidaya, Lee, Burstyn, Yudell, Mortensen and Newschaffer23,Reference Castro, Kong, Clements, Brady, Kaimal and Doyle30,Reference Croen, Grether, Yoshida, Odouli and Hendrick31
Table 1 Characteristics of included studies
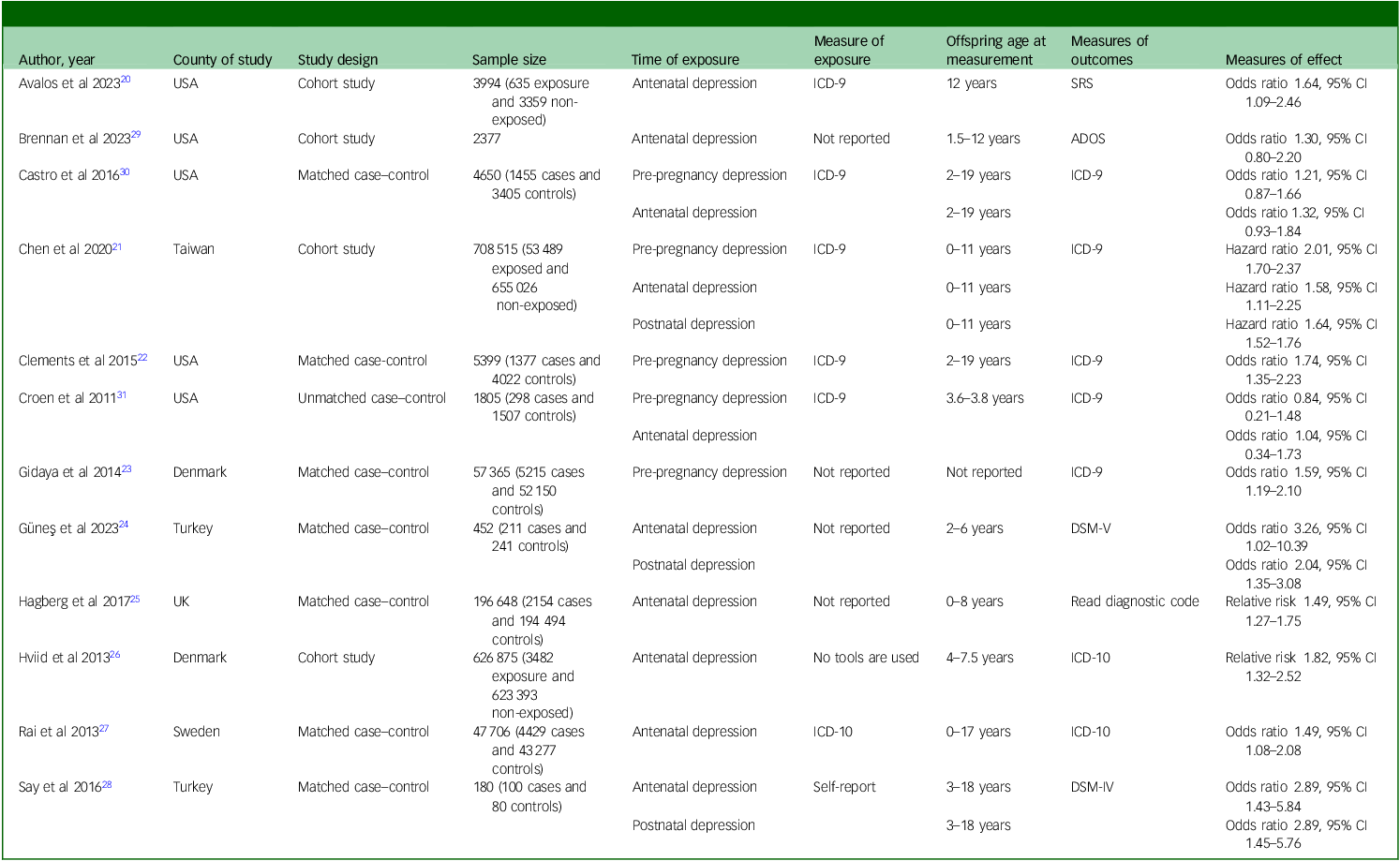
SRS, Social Responsiveness Scale; ADOS, Autism Diagnostic Observation Schedule; ICD, International Classification of Diseases; DSM, Diagnostic and Statistical Manual of Mental Disorders.
Adjustment of confounder factors
Of the 12 studies, nine accounted for at least one confounding factor in their effect estimates. Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Clements, Castro, Blumenthal, Rosenfield, Murphy and Fava22,Reference Hagberg, Robijn and Jick25–Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27,Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29–Reference Croen, Grether, Yoshida, Odouli and Hendrick31 Eight studies considered the impact of socioeconomic factors Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Clements, Castro, Blumenthal, Rosenfield, Murphy and Fava22,Reference Hviid, Melbye and Pasternak26,Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27,Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29–Reference Croen, Grether, Yoshida, Odouli and Hendrick31 and six adjusted for maternal substance use. Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20,Reference Hagberg, Robijn and Jick25–Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27,Reference Brennan, Dunlop, Croen, Avalos, Salisbury and Hipwell29,Reference Castro, Kong, Clements, Brady, Kaimal and Doyle30 Additionally, five studies accounted for antidepressant use, and four controlled for other psychiatric disorders. Reference Hagberg, Robijn and Jick25–Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27,Reference Castro, Kong, Clements, Brady, Kaimal and Doyle30 Notably, only two studies included adjustments for paternal depressive disorders (see Supplementary Table 3). Reference Chen, Chen, Hsu, Huang, Bai and Chen21,Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27
Quality assessment
All included studies were rated medium to high based on the NOS quality assessment, with a median score of 8 (interquartile range: 2) points. Eight studies achieved scores of eight or nine points, indicating high quality, Reference Avalos, Chandran, Churchill, Gao, Ames and Nozadi20–Reference Clements, Castro, Blumenthal, Rosenfield, Murphy and Fava22,Reference Hviid, Melbye and Pasternak26–Reference Croen, Grether, Yoshida, Odouli and Hendrick31 whereas the remaining four received scores of 7 points, falling within the category of medium-quality studies (see Supplementary Table 4). Reference Gidaya, Lee, Burstyn, Yudell, Mortensen and Newschaffer23–Reference Hagberg, Robijn and Jick25
Maternal depression and risk of ASD
Among the 12 studies examining the link between maternal pre- and perinatal depression and the subsequent risk of ASD, nine recorded significant relationships, whereas the remaining three found no evidence of associations. A random-effects meta-analysis of these studies revealed a 52% increased risk (odds ratio 1.52, 95% CI 1.13–1.90) of ASD in the offspring of mothers experiencing pre-pregnancy depression, a 48% increased risk (odds ratio 1.48, 95% CI 1.32–1.64) in those experiencing antenatal depression, and a 70% increased risk (odds ratio 1.70, 95% CI 1.41–1.99) in those experiencing postnatal depression (Fig. 2).
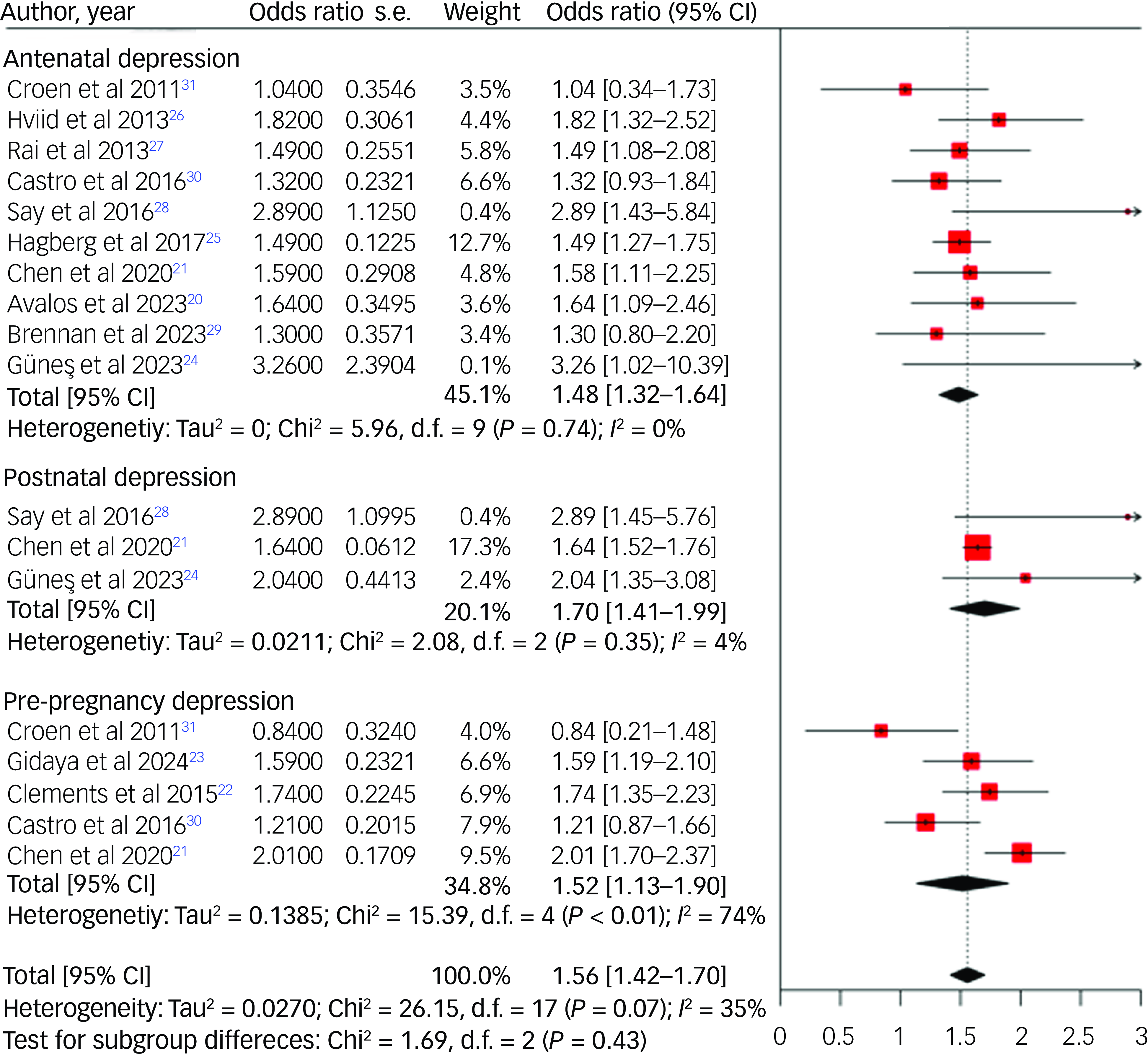
Fig. 2 Forest plot for risk of autism spectrum disorder among offspring of mothers with depression.
Subgroup analysis
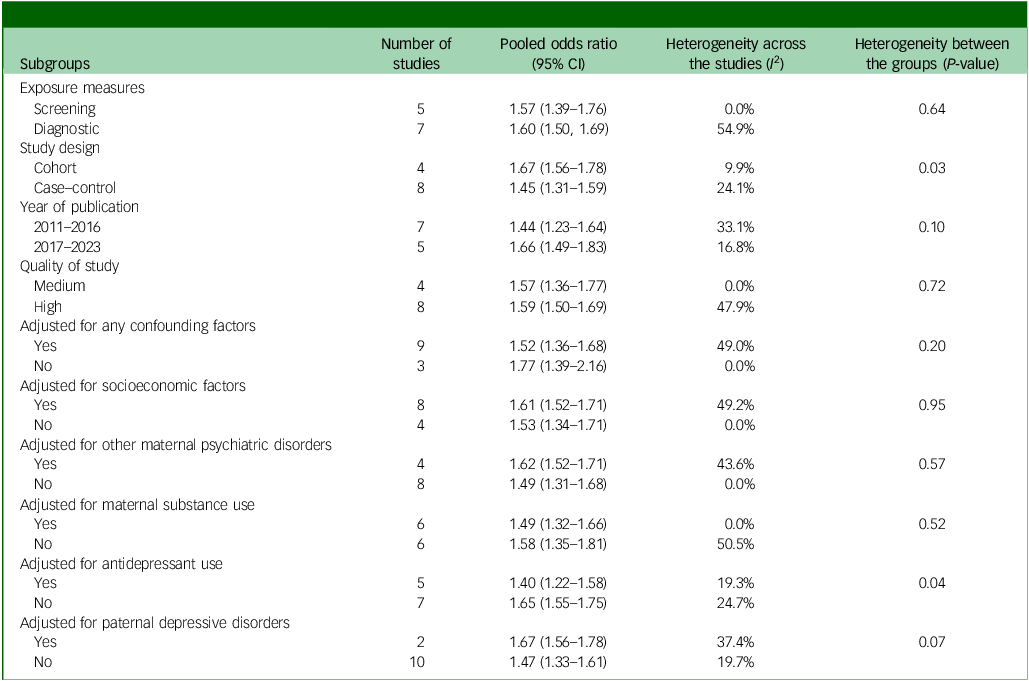
A stratified analysis based on study design indicated a higher risk of offspring ASD in cohort studies (odds ratio 1.67, 95% CI 1.56–1.78) compared with case–control studies (odds ratio 1.45, 95% CI 1.31–1.59). Additionally, our subgroup analysis identified a greater risk of offspring ASD in studies published between 2017 and 2023 (odds ratio 1.66, 95% CI 1.49–1.83) compared with those from 2011–2016 (odds ratio 1.44, 95% CI 1.23–1.64). Positive associations between maternal depression and ASD in offspring were noted in studies employing both diagnostic instruments (odds ratio 1.60, 95% CI 1.39–1.76) and screening instruments (odds ratio 1.57, 95% CI 1.39–1.76). Similarly, a positive association was evident in both high-quality (odds ratio 1.59, 95% CI 1.50–1.69) and medium-quality studies (odds ratio 1.57, 95% CI 1.36–1.77).
The risk of ASD was slightly lower in studies that accounted for at least one confounding factor (odds ratio 1.52, 95% CI 1.36–1.68) compared with those that did not (odds ratio 1.77, 95% CI 1.39–2.16). Specifically, the risk of offspring ASD exhibited a slight decrease in studies that accounted for the effects of socioeconomic factors (odds ratio 1.61, 95% CI 1.52–1.71 v. odds ratio 1.53, 95% CI 1.34–1.71), other maternal psychiatric disorders (odds ratio 1.62, 95% CI 1.52–1.71 v. odds ratio 1.49, 95% CI 1.31–1.68), maternal substance use (odds ratio 1.49, 95% CI 1.32–1.66 v. odds ratio 1.58, 95% CI 1.35–1.81) and maternal antidepressant use (odds ratio 1.40, 95% CI 1.22–1.58 v. odds ratio 1.65, 95% CI 1.55–1.7) compared with those that did not adjust for these factors. Only two studies accounted for paternal depression in their analysis. These studies reported a higher risk of ASD (odds ratio 1.67, 95% CI 1.56–1.78) compared with those that did not account for paternal depression (odds ratio 1.47, 95% CI 1.33–1.61). It is important to note that both of these studies exhibited high ratings in the NOS quality assessment, utilised substantial sample sizes and employed validated diagnostic tools (ICD-9/10) to assess the outcome (ASD) and exposure (maternal depression). These methodological strengths likely contributed to the higher risk estimates observed in this subset of studies (Table 2).
Table 2 Subgroup analysis on risk of autism spectrum disorder in offspring of mothers with depression
Publication bias
The symmetrical funnel plots and Egger’s test indicate no evidence of substantial publication bias among the included studies (P = 0.92) (Fig. 3).
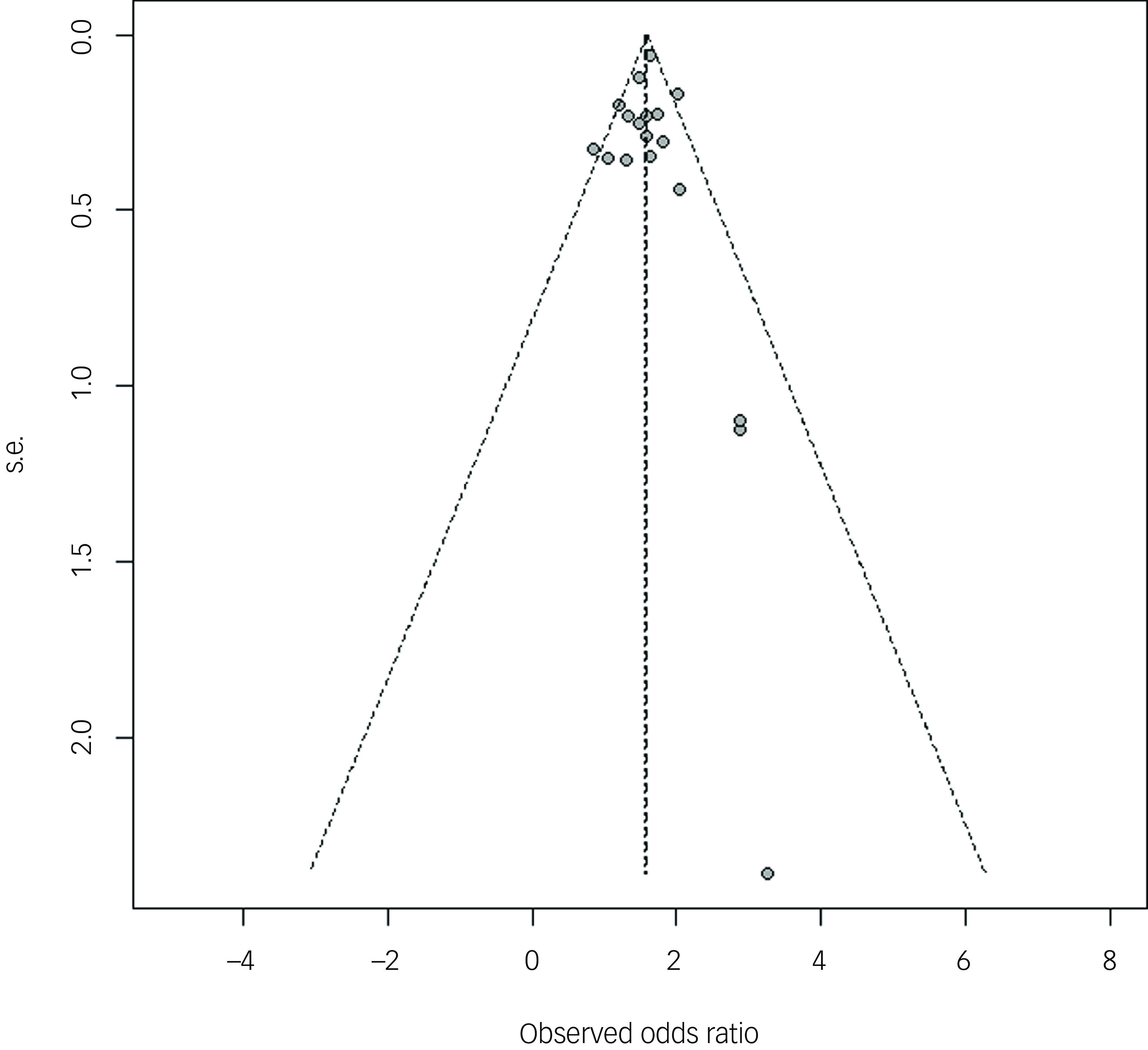
Fig. 3 Publication bias of studies.
Discussion
In our systematic review and meta-analysis, we investigated the potential association between maternal pre- and perinatal depression and the risk of ASD in offspring, based on 12 studies involving over 1.6 million mother–offspring pairs. We found that offspring exposed to maternal pre-conception, antenatal and postnatal depressions exhibited a 48–70% increased risk of ASD. The observed associations were independent of methodological factors such as ascertainment of exposure and outcome, year of publication, study quality and adjustment for important potential confounders, including socioeconomic status, maternal substance use, comorbid maternal psychiatric disorders, paternal depression and maternal antidepressant use.
Although the exact mechanism elucidating the observed associations between pre- and perinatal depression and the elevated risk of ASD in offspring remains unclear, several interpretations have been proposed. First, it is suggested that there may exist a shared genetic predisposition between depression and ASD, where certain genetic variations or mutations could contribute to both conditions, thus increasing the probability of their co-occurrence within families. Reference Zhao and Nyholt11 Second, epigenetic factors, such as alterations in DNA methylation patterns, might play a role in mediating the relationship between maternal depression and the risk of ASD in offspring, implying environmental influences on gene expression. Reference Nemoda and Szyf18 Maternal depression during pregnancy could potentially affect DNA methylation of the fetus through changes in maternal stress hormones, inflammatory cytokines or neurotransmitter levels. Reference Christian, Franco, Glaser and Iams14,Reference Talge, Neal and Glover17,Reference Oberlander, Weinberg, Papsdorf, Grunau, Misri and Devlin19 These alterations could subsequently affect the developing fetal brain, thereby elevating the risk of ASD in the offspring. Reference Kinney, Munir, Crowley and Miller15–Reference Talge, Neal and Glover17
Maternal behaviour and caregiving practices may explain the observed associations between maternal postpartum depression and ASD risk. Maternal depression can affect the quality of maternal–child interactions, Reference Śliwerski, Kossakowska, Jarecka, Świtalska and Bielawska-Batorowicz39 potentially worsening existing ASD symptoms in children. Reference Loncarevic, Maybery, Barbaro, Dissanayake, Green and Hudry40 For instance, women who experience depression often face difficulties in bonding with their children or find it more challenging to respond to the added needs of children at risk of ASD. Reference Śliwerski, Kossakowska, Jarecka, Świtalska and Bielawska-Batorowicz39 These challenges can extend to breastfeeding, as the emotional connection and interaction between mother and baby play a crucial role in successful breastfeeding. Reference Kim, Park and Ahn41 Consequently, suboptimal breastfeeding practices have been associated with the worsening of existing ASD symptoms in children. Reference Ghozy, Tran, Naveed, Quynh, Helmy Zayan and Waqas42 In addition, postpartum depression is often associated with chronic stress and dysregulated hypothalamic-pituitary-adrenal axis function, leading to elevated cortisol levels. Reference Glynn, Davis and Sandman43,Reference Handley, Dunn, Waldron and Baker44 Exposure to high levels of maternal cortisol during early infancy can affect the developing child’s stress response systems and neural circuits involved in emotional regulation and social behaviour, potentially increasing the risk of ASD. Reference Makris, Agorastos, Chrousos and Pervanidou45 Postpartum depression is associated with immune dysregulation and increased inflammation. Reference Bränn, Fransson, White, Papadopoulos, Edvinsson and Kamali-Moghaddam46 Maternal inflammatory markers and cytokines can also transmit through breast milk, exposing the infant to an inflammatory environment that may affect brain development and increase the risk of neurodevelopmental disorders, including ASD. Reference Jiang, Cowan, Moonah and Petri47,Reference Patterson48 In light of the above mechanisms, although the 95% confidence intervals for all three types of maternal depression overlap, suggesting that these differences are not statistically significant, the effect estimate is slightly higher for postnatal depression compared with pre-pregnancy and antenatal depression. Although antenatal depression is critical because of its potential effects on early brain development in utero, postnatal depression may exert additional influence through altered caregiving behaviours, disrupted maternal–infant bonding and increased environmental stressors during a crucial period of rapid brain growth and neural plasticity. Furthermore, postnatal depression may represent a continuation or exacerbation of earlier depressive states, compounding the overall risk to the child. Additionally, the number of studies included in the analyses could contribute to variability in the effect estimates. Although five studies were included for pre-pregnancy depression and ten for antenatal depression, only three studies were available for postnatal depression.
Another plausible explanation for the association between maternal depression and the risk of ASD is the potential confounding effect of maternal antidepressant use during pregnancy. Antidepressants, particularly selective serotonin reuptake inhibitors (SSRIs) and serotonin/noradrenaline reuptake inhibitors (SNRIs) can traverse the placental barrier. Reference Horackova, Karahoda, Cerveny, Vachalova, Ebner and Abad49 Research indicates that exposure to serotonergic agents in utero can induce enduring alterations in brain circuitry, diminish serotonergic reactivity and manifest behavioural traits akin to autism in animal models. Reference Rodriguez-Porcel, Green, Khatri, Harris, May and Lin50 Moreover, pregnant women who take SSRIs /SNRIs are more likely to have frequent medical consultations during and after pregnancy compared wuth those who do not. Consequently, their children may also have more medical encounters, potentially leading to a higher likelihood of ASD detection compared with children of non-exposed mothers. Our study’s estimates were notably influenced by adjustment for this factor, suggesting its significance in interpreting the association. A systematic review and meta-analysis comprising more than 100 000 mother–offspring pairs reported a positive association between antidepressant exposure and the risk of ASD. Reference Mezzacappa, Lasica, Gianfagna, Cazas, Hardy and Falissard51 In our meta-analysis, we observed a notable decrease in the risk of ASD in studies that accounted for maternal antidepressant use, indicating a potential influence of these exposures on the observed association. It is also plausible that if women effectively manage their depression with antidepressants, they may be better equipped to handle their children’s condition, potentially leading to a reduced risk of ASD. However, this adjustment did not completely attenuate the association, suggesting that maternal antidepressant may have contributed only partly to the increased risk of ASD in offspring.
Socioeconomic factors, such as maternal age, maternal education status and family income, alongside substance use behaviours such as alcohol and cigarette consumption, are also significant confounders in the relationship between maternal depression and the risk of offspring ASD. Reference Zhang, Zhang, Li and Jiang52–Reference Francis55 Research has shown a link between maternal depression and lower socioeconomic status, Reference Zhang, Zhang, Li and Jiang52 with low socioeconomic status itself being associated with an increased risk of ASD in offspring. Reference Wu, Wu, Ding, Hou, Bi and Zhang53,Reference Yu, Lien, Liang and Kuo54 This relationship may be explained by the exposure of mothers to social stressors associated with low socioeconomic status, particularly during pregnancy or childbirth, which could affect ASD risk through epigenetic mechanisms. Reference Kinney, Munir, Crowley and Miller15,Reference Talge, Neal and Glover17 Furthermore, factors associated with lower socioeconomic status, such as lead poisoning, exposure to other neurotoxins and alcohol consumption during pregnancy, could also contribute to this relationship. Reference Patterson48 Existing epidemiology studies suggested that substance use is often associated with maternal depression, Reference Hasin and Kilcoyne56 and this substance use can have harmful effects on fetal brain development and increase the risk of ASD. Reference Francis55 Consistent with this, our meta-analysis revealed a slight decrease in ASD risk in studies that accounted for socioeconomic factors and substance use, suggesting a potential influence of these variables on the observed association. However, even after adjusting for these factors, the link between maternal depression and the risk of offspring developing ASD persisted.
There are also likely additional factors that could significantly influence the association between maternal depression and the risk of offspring ASD. However, these factors have not been adequately controlled for in most of the included studies. For example, of the 12 studies in our meta-analysis, only two adjusted for paternal depression. Reference Chen, Chen, Hsu, Huang, Bai and Chen21,Reference Rai, Lee, Dalman, Golding, Lewis and Magnusson27 However, it is important to note that our subgroup analyses revealed positive associations in both studies that did and did not account for paternal depression, although the effect estimate is slightly higher for the former one. The difference in study design, sample size, study quality and ascertainment of exposure and outcome may have contributed to this disparity.
Strengths and limitations of the study
This systematic review and meta-analysis includes several noteworthy strengths. It investigates the relationship between maternal pre- and perinatal depression, and the likelihood of ASD in offspring, offering a comprehensive overview of existing research. The exploration of sources of heterogeneity through subgroup analyses enhances the study’s credibility by identifying potential variations. Moreover, the high scores on the NOS for most included studies reflect a high methodological quality among the reviewed studies.
Despite its strengths, the study has notable limitations. Variations in how maternal depression were assessed may introduce errors in risk estimation, affecting validity. Although no evidence of publication bias was found, potential bias resulting from unpublished research remains. Additionally, several potential confounders, such as parental neurodevelopmental disorders, maternal physical comorbidities, prenatal health conditions and obstetric complications, were not accounted for in the majority of studies. As a result, the observed association between maternal depression and the increased risk of ASD in offspring must be interpreted with caution because of the insufficient or absent adjustment for these important factors in most included studies. Future research should address these and other unmeasured confounders to provide a more thorough and accurate understanding of the relationships. Moreover, given that the association between postnatal depression and the risk of ASD observed in this meta-analysis is based on only three studies, further research is needed to strengthen the evidence. Finally, the generalisability of findings may be limited as many studies were conducted in developed countries, limiting applicability to developing countries with different healthcare systems, cultural factors and socioeconomic conditions.
In conclusion, our systematic review and meta-analysis have revealed an elevated risk of ASD in the offspring of mothers who experience pre-and perinatal depression. Therefore, early screening and targeted intervention programmes for these at-risk children and adolescents are essential.
Supplementary material
The supplementary material can be found online at https://doi.org/10.1192/bjo.2025.67
Data availability
The data that support the findings of this study are available from the corresponding author, B.T., upon reasonable request.
Acknowledgements
We express our gratitude to Curtin University for granting us access to a diverse array of online databases. B.T. is the recipient of the Curtin University Higher Degree Research (HDR) Scholarship and extends appreciation to Curtin University for its generous support.
Author contributions
B.T., B.D., R.A. and G.A. conceptualised the study hypothesis. B.T., B.D., G.A. and A.B.W. performed data curation, access, validation and statistical analysis. B.T. drafted the first version of the manuscript, with input from B.D., G.A., K.B. and R.A. All authors collaboratively reviewed and approved the final manuscript in terms of its intellectual content.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors
Declaration of interest
None.









eLetters
No eLetters have been published for this article.