A high-quality diet has been found to reduce the odds of major depressive disorder (MDD) in various observational studies carried out across countries and different age groups. The most substantial evidence supports the Mediterranean diet, although there is also evidence that avoiding a pro-inflammatory diet provides some protection against depressive symptoms and MDD(Reference Lassale, Batty and Baghdadli1). In addition, there has been an increasing interest in dietary interventions as an adjunctive component of treating MDD. Nutritional counselling and improvements in diet quality are well justified, as people with clinical depression also face a higher risk of obesity(Reference Pereira-Miranda, Costa and Quieros2) and cardiometabolic diseases, such as type 2 diabetes(Reference Semenkovich, Brown and Svrakic3) and CVD(Reference Penninx4), in which diet plays a significant role both in terms of risk and prevention(Reference Berk, Köhler-Forsberg and Turner5). A meta-analysis examining the efficacy of dietary interventions found a small positive effect on depressive symptoms(Reference Firth, Marx and Dash6). However, nearly all previous trials have investigated the impact of dietary interventions in samples with non-clinical depression. Their focus has been on dietary changes to induce weight loss rather than to improve diet quality.
According to the most recent meta-analysis, the Mediterranean diet significantly reduces depressive symptoms in young and middle-aged adults with MDD or mild to moderate depressive symptoms compared to controls(Reference Bizzozero-Peroni, Martínez-Vizcaíno and Fernández-Rodríguez7). To our knowledge, there are only four randomised controlled trials (RCT) published with adults with a diagnosis of MDD. All these interventions have been at 12 weeks(Reference Jacka, O’Neil and Opie8–Reference Radkhah, Rasouli and Majnouni11). The nature and intensity of nutrition counselling varied: the SMILES study(Reference Jacka, O’Neil and Opie8) had seven 1-hour individual sessions with a clinical dietitian, and the AMMEND study(Reference Parletta, Zarnowiecki and Cho9) had three sessions with a clinical nutritionist, whereas the HELFIMED study participants had six dietitian-led group counselling sessions that also included activities like cooking and shopping(Reference Bayes, Schloss and Sibbritt10). In the most recent RCT, the intervention group had one session at the beginning of the study to receive specific instructions on how to follow a Mediterranean diet(Reference Radkhah, Rasouli and Majnouni11). All these RCT focused on the Mediterranean diet, supplemented with fish oil in the HELFIMED trial(Reference Bayes, Schloss and Sibbritt10). Positive effects of the interventions on diet quality(Reference Jacka, O’Neil and Opie8–Reference Bayes, Schloss and Sibbritt10) and depressive symptoms(Reference Jacka, O’Neil and Opie8–Reference Radkhah, Rasouli and Majnouni11) were reported, even though the changes in depressive symptoms were not clinically significant in the study by Radkhah et al.(Reference Radkhah, Rasouli and Majnouni11). Although the initial results of studies focusing on the efficacy of dietary interventions have been promising, there has been little discussion about translating the findings into real-world settings and clinical practice and the need for tailoring the interventions to fit the realities of routine care(Reference Skivington, Matthews and Simpson12). According to the latest update of the British Medical Research Council guidance on developing and evaluating complex interventions, examining the feasibility and acceptability of the interventions is recommended before developing extensive complex intervention studies(Reference Skivington, Matthews and Simpson12). Feasibility refers to how a new treatment or innovation can be successfully implemented in a specific organisational context or setting(Reference Proctor, Silmere and Raghavan13). Feasibility considerations help to identify potential enablers and barriers that may arise during the study and enable the optimisation and practicality of the intervention(Reference Skivington, Matthews and Simpson12). Acceptability is defined as the perception of implementation stakeholders (such as patients, healthcare professionals and service providers) of the examined treatment or innovation as agreeable, palatable or satisfactory(Reference Proctor, Silmere and Raghavan13). Acceptability among patients has been linked to treatment adherence, engagement and satisfaction(Reference Proctor, Silmere and Raghavan13). As a result, it may also contribute to improved treatment outcomes and overall well-being, and foster a patient-centred approach to healthcare(Reference Sekhon, Cartwright and Francis14). Incorporating feasibility and acceptability into a healthcare intervention design influences practicality, real-world effectiveness, sustainability, scalability and ethical appropriateness(Reference Klaic, Kapp and Hudson15).
Prior RCTs have analysed a range of secondary outcomes such as adherence to diet(Reference Opie, O’Neil and Jacka16), improvements in knowledge and skills related to recommended meals, dietary habits and biomarkers(Reference Opie, O’Neil and Jacka16) and participant-reported changes in diet habits and cooking skills(Reference Bogomolova, Zarnowiecki and Wilson17). However, feasibility has only been evaluated regarding participant-reported experiences, challenges and the benefits of adhering to a Mediterranean diet(Reference Bayes, Schloss and Sibbritt18). None of the trials have assessed the acceptability of diet intervention in the treatment of depression.
Our objective was to develop a group-based brief dietary intervention model that could be implemented as a part of mental health care in Finland(Reference Roponen, Ruusunen and Absetz19). This pilot study, involving two parallel group interventions, aims to evaluate the model by examining feasibility through (1) the reach and engagement of the participants, (2) appropriateness and acceptability of the interventions among the participants and (3) the short-term effect of the interventions on diet quality and depressive symptoms.
Materials and methods
The data are part of the randomised controlled Food for Mind (FM) trial conducted in Finland from February 2018 to December 2021. The detailed protocol of the FM study was published earlier(Reference Roponen, Ruusunen and Absetz19). The participant flow and study procedures of this study are described in Figure 1.
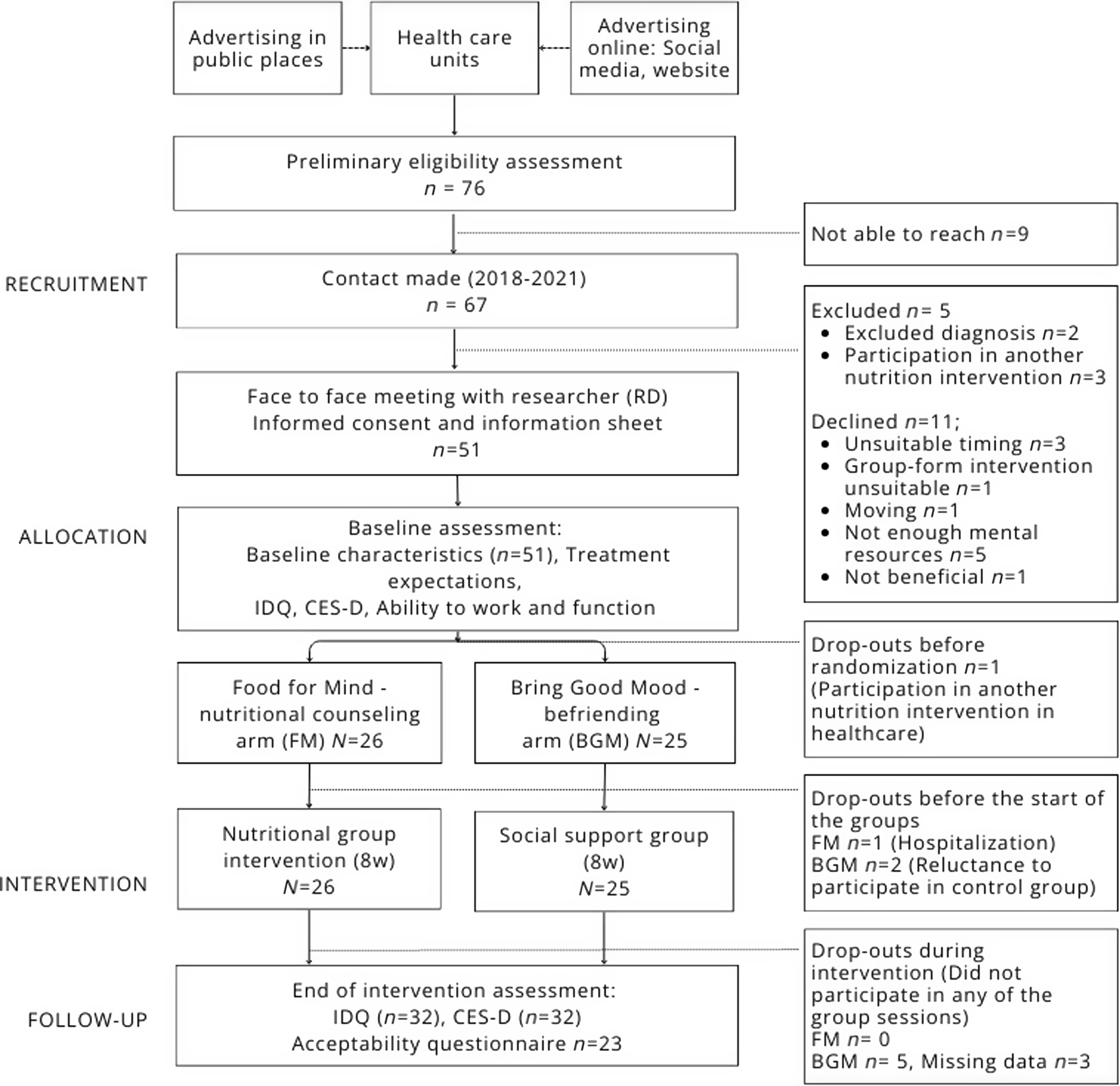
Figure 1. Participant flow and study procedures of Food for Mind intervention study. IDQ, Index of Diet Quality; CES-D, The Center for Epidemiologic Studies Depression Scale; FM, Food for Mind; BGM, Bring Good Mood.
Recruitment
Data were collected during a 40-month period, from February 2018 to June 2021. Participants (n 51) were recruited in collaboration with ten public and private healthcare service provider organisations in the North Savo region in Finland. The healthcare staff identified potentially eligible patients, introduced the study to them and shared the contact information of the consenting patients with the research team for further eligibility screening. To raise awareness of the study, posters and flyers were displayed and distributed in healthcare units, public libraries and cafés. Information on the study was also available online and on social media (Facebook). Social media ads and emails were utilised in collaboration with local mental health associations.
Patients were considered eligible if they (1) were 20–65 years old, (2) had the ICD-10 diagnosis for depressive disorder (F32·1, F32·2, F32·8, F32·9, F33·1, F33·2, F33·8, F33·9 or F34·1), (3) had an individually tailored treatment plan from a healthcare unit, (4) were receiving ongoing treatment (medication and/or psychotherapy) that had not changed for at least 2 weeks prior to the randomisation and (5) were willing to participate in six group sessions during an 8-week intervention period. Patients were excluded if they (1) had a clinically unstable medical illness, which could be aggravated by the intervention, (2) were pregnant, (3) were currently participating in another nutrition or exercise intervention or (4) had a personality disorder, a severe eating disorder, or a substance use disorder or had a current depressive episode with psychotic features or a recurrent depressive disorder with psychotic features.
After the patients gave their informed consent to participate, baseline assessments were conducted. The participants were randomised into the FM nutrition intervention arm facilitated by a registered dietitian or the Bring Good Mood (BGM) social support arm, which was facilitated by a rehabilitation counsellor from a non-profit organisation (allocation ratio 1:1). A random study number and a number coding the treatment was generated with Microsoft Excel for each study participant by a data manager not connected to the study. Randomisation was done separately for each block (n 8–12) after obtaining the participants’ consent and conducting baseline assessments. The interventions were implemented in small groups of 4–6 participants. In both arms, the groups participated in six sessions (5 × 1·5 h and 1 × 3 h) during an 8-week period, and a closed social media (WhatsApp) group was used for online social support. These online support groups were formed for both arms and facilitated by a registered dietitian. In the nutrition intervention arm, the dietitian assigned one task for the online support group after each session to enhance engagement in the intervention and to enable peer support. In the BGM arm, the online support group was used to promote adherence to group sessions and prevent drop-outs. In both arms, the dietitian also monitored possible harmful online behaviours on WhatsApp. End-of-intervention assessments were conducted using questionnaires participants filled out at home after the intervention (at eight weeks).
Intervention arms
The Food for Mind arm
The main aim of behavioural nutrition counselling was to improve diet quality based on the ‘Food for Health’ Finnish Nutrition and Food Recommendations(20). According to these recommendations, a healthy diet includes vegetables, fruits and berries, whole-grain products, legumes, fish, vegetable oils, vegetable oil spreads, nuts and seeds and fat-free or low-fat dairy products. A high-quality diet includes low energy density, fulfils nutrient requirements and is rich in bioactive compounds. Alongside nutritional quality, importance is given to a regular meal frequency consisting of breakfast, lunch, dinner and two snacks(20).
Behavioural nutrition counselling adopted a strength-based and patient-centred approach, emphasising equality and empowerment(Reference Angle21). It was based on positive psychology(Reference Van Cappellen, Rice and Catalino22) and the self-determination theory(Reference Deci and Ryan23) and utilised practical strategies from Motivational Interviewing(Reference Copeland, McNamara and Kelson24) and Solution-Focused Therapy(Reference Cheng25), which were described in more detail previously(Reference Roponen, Ruusunen and Absetz26).
Counselling was provided in the group sessions and included activities and assignments designed to enhance the participants’ sense of autonomy, competence and relatedness(Reference Roponen, Ruusunen and Absetz19). Each session included discussions on the topic and action-based methods, such as grocery shopping and cooking. In addition to the sessions, the participants were given home assignments to improve their daily diet quality. At the start of each session, the participants shared feedback on their experiences and positive changes in their eating habits. Printed counselling material and topic-related assignments were provided to help the participants observe their eating behaviour, practice mindful eating and identify hunger, satiety and emotions. A detailed description of the group sessions has been published elsewhere(Reference Roponen, Ruusunen and Absetz26).
The Bring Good Mood arm
To control for the effect of peer support on mental health outcomes, the comparison group received a group-based intervention program with a befriending protocol, which is widely used in psychological intervention studies(Reference Bendall, Jackson and Killackey27). The participants’ schedule was identical to that of the FM arm in terms of quantity and duration. The group sessions of the BGM social support intervention arm included discussing neutral topics, such as hobbies, music, sports and shared activities. The purpose of these interactions was to keep the participants engaged and maintain a positive mood throughout the sessions.
Study measures
Feasibility
To assess the feasibility(Reference Orsmond and Cohn28) of the FM study, 1) reach and engagement, 2) appropriateness and 3) the acceptability of the intervention and study procedures were evaluated.
Reach and engagement
Data regarding the recruitment capability, reasons for exclusion and reasons to decline participation were collected from participant flow data and baseline assessments. The engagement of the intervention and study procedures were assessed with retention and adherence using intervention attendance logs tracked by the group facilitators and participation at the end-of-intervention assessment.
Appropriateness
In addition, a questionnaire was used to assess whether the therapeutic group conditions favourable to a lifestyle change were established during the intervention. At the end of the final group session, the participants filled out the questionnaire with five statements adapted from Sharry(Reference Sharry29): (1) the relevance of the intervention content to participants’ needs and goals, (2) the feeling of being understood and supported, (3) the feeling of being engaged and active, (4) the feeling of being hopeful about one’s progress in the intervention and the (5) facilitator’s success in maintaining effective group engagement (FM arm n 13, BGM arm n 10). The statements were scaled from 1 (completely disagree) to 5 (completely agree), with a higher score indicating higher success in establishing therapeutic conditions.
Acceptability
The acceptability of the FM arm and BGM arm was evaluated retrospectively using a self-administered questionnaire based on the theoretical framework of acceptability (TFA)(Reference Sekhon, Cartwright and Francis30). The framework considers the appropriateness of the intervention from the perspective of those involved (e.g. patients and healthcare professionals), taking their cognitive and emotional responses into account. Sekhon’s framework categorises acceptability into anticipated and experiential acceptability. In our study, we employed the experiential approach. The 7-component construct of acceptability, according to Sekhon et al. (2017), is represented in Figure 2.
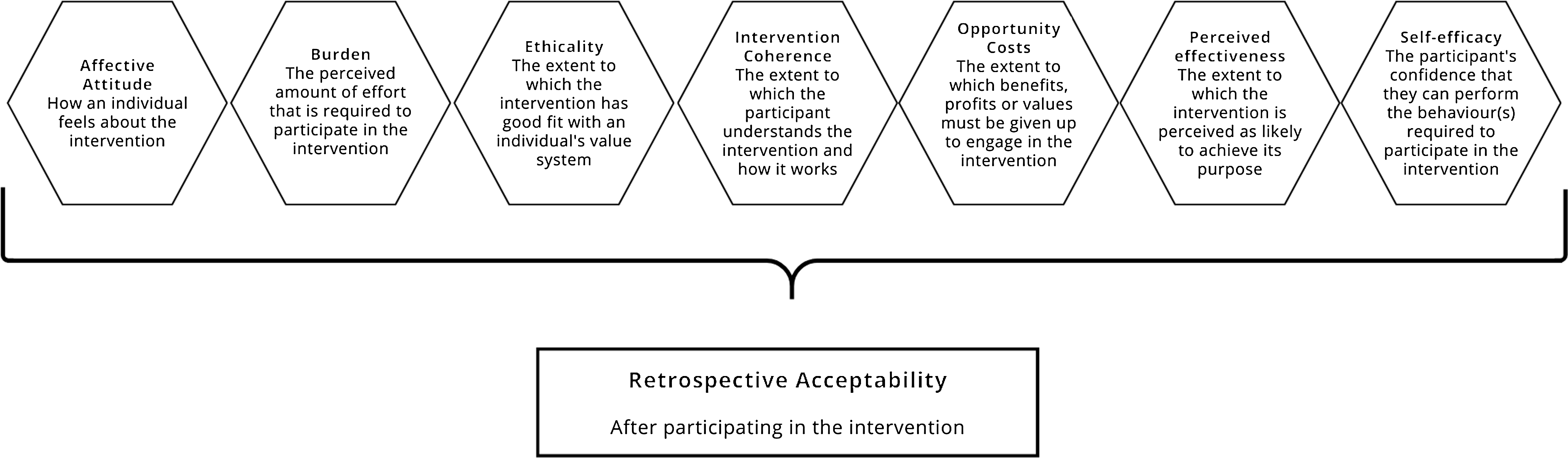
Figure 2. The components of the Theoretical Framework of Acceptability of Healthcare Intervention (adapted from Sekhon et al. 2017(Reference Sekhon, Cartwright and Francis30)) applied in the Food for Mind study.
The acceptability questionnaire based on the TFA was administered at the end of the last small group session. The questionnaire consisted of seven statements, one per framework component, scaled from 1 (completely disagree) to 5 (completely agree). The statement assessing opportunity costs was reverse scored, so a higher score always indicated higher acceptability. The total acceptability score was calculated by summing up the scores for each statement and dividing them by the number of statements(Reference Sekhon, Cartwright and Francis14).
Short term effects
Diet quality
Diet quality was assessed with a validated Finnish Index of Diet Quality (IDQ) questionnaire(Reference Leppälä, Lagström and Kaljonen31,Reference Mäkelä32) at baseline and the end of the intervention. The IDQ assessed adherence to health-promoting dietary guidelines through eighteen questions regarding the consumption of whole grains, fats, vegetables, fruits, sugar and dairy products and adherence to a regular meal frequency. Based on responses, a dietary score from 0 (zero) to 15 was calculated, with a score of 10 or higher indicating a healthy diet. The IDQ was used as a continuous variable without a specific cut-off to differentiate between healthy and unhealthy diets in the analysis.
Depressive symptoms
All participants had a current diagnosis of depressive disorder assessed by a medical doctor, and their depressive symptoms were assessed using a self-report tool, the Center for Epidemiologic Studies Depression (CES-D) Scale(Reference Radloff33) at baseline and the end of the intervention. The scale consists of twenty items, each scored from 0 to 3, resulting in a total score ranging from 0 to 60. Four items (#4, #8, #12, #16) are reversed before calculating the total score. The total score was not calculated if more than five items had missing information. Otherwise, the sum variable was calculated by dividing the sum of answered items by the number of answered items and multiplying by 20. A higher total score indicates a more significant presence of depressive symptoms.
Other measures
As treatment expectations might influence the intervention outcome, the participants’ expectations were evaluated with the six-item Treatment Expectancy Questionnaire(Reference Devilly and Borkovec34) at baseline. This questionnaire examined participants’ beliefs regarding the effectiveness of the intervention. Four questions were related to thinking, while two questions concerned feelings. Standardised scores were utilised to account for the use of two different rating scales, ranging from 1 to 9 and 0 to 100 %.
To consider seasonal changes in mood, appetite, weight, sleep duration, social activity and energy level, the CES-D was supplemented with a Global Seasonality Score (GSS) at baseline. GSS was derived from a modified version(Reference Pajunen, Lönnqvist and Partonen35) of the original Seasonal Pattern Assessment Questionnaire(Reference Piiroinen and Partonen36,Reference Rosenthal, Sack and Gillin37) , a self-report tool that evaluates the extent of seasonal change in mood.
Sex, marital status (living alone/married or cohabitating), level of education (primary or upcondary level/lower or higher tertiary level), employment status (working or studying part-time or full-time/long-term sick leave or disability pension due to depression/unemployed or laid off), medications and the perceived work ability compared to the lifetime best were inquired with a background questionnaire at baseline. The participants also reported their prescriptions, if any.
Statistical analyses
Considering the exploratory nature of this feasibility study, a formal power calculation was not performed. The sample size was compliant with the standard practices for similar studies(Reference Bell, Whitehead and Julious38). The power calculation for the effectiveness trial has been published elsewhere(Reference Roponen, Ruusunen and Absetz19).
Baseline characteristics and assessment of the acceptability and establishment of therapeutic conditions during the intervention were analysed using SPSS 27.0 for Windows(39). The independent samples t test was used for continuous variables, and the chi-squared test was used for categorical variables to assess differences in baseline characteristics between FM and BGM arms.
For the analyses, missing values from continuous variables (work ability compared to lifetime best n 9, GSS n 8, treatment expectations; credibility n 9, expectancy n 9) were replaced with sample means and categorical variables (medication for MDD n 7, diabetes medication n 8, cardiovascular medication n 8, marital status n 7, educational level n 7 and work status n 7) were replaced with the most common answer. Missing values in IDQ (baseline n 9, 8 weeks n 18) or CESD (baseline n 8, 8 weeks n 18) were not imputed. We performed multiple imputations to account for the missing values (FM arm n 5, BGM arm n 2) of the ‘perceived effectiveness’ dimension in Sekhon’s Theoretical Framework of Acceptability, generating five imputed datasets. We used pooled means to evaluate the differences in the ‘perceived effectiveness’ dimension between the FM and BGM arms, performed Mann–Whitney U tests for the five imputed datasets and used Fisher’s method(Reference Fisher40) to combine P-values to evaluate the statistical significance. Similar methods were used to analyse the differences in the ‘total’ acceptability between the FM and BGM arms. The establishment of therapeutic conditions during the intervention was analysed using the Mann–Whitney U test.
The effects of the intervention on diet quality and depressive symptoms were analysed following the intention-to-treat principle. Analyses were performed with R Statistical Software version 4.3.1(41) and RStudio, Integrated Development Environment for R, version 2023·06·1 + 524(42). Group differences were estimated with linear mixed-effects models using the lme4 package (version 1.1–34)(Reference Bates, Mächler and Bolker43). Models were fitted using the restricted maximum likelihood method. Contrary to the original study protocol(Reference Roponen, Ruusunen and Absetz19), the random effect of the nested dependency structure between groups (subgroups) and group facilitators was not considered in the final models, as the effect either did not manifest in models or its impact was negligible.
Linear mixed model analyses were used to study the treatment effect (study group × time-point, measured in weeks, interaction) on the outcome of interest (IDQ and CES-D). Along with the treatment effect, models were adjusted for potential confounders chosen based on the original study protocol and previous literature. The main models included the outcome of interest (IDQ or CES-D) as a dependent variable, study group (FM/BGM), timepoint (baseline, 8 weeks), sex, age, depression medication, marital status, educational level, employment status, treatment expectations (Credibility and Expectancy scores) and GSS as fixed effects; and the subject identifier as a random effect. The CESD model was further adjusted for diet quality (IDQ) and vice versa. All tests were conducted using an alpha level of 0·05 and reporting 95 % CI.
Results
Participant characteristics
Most of the participants were women and had completed either primary or upper-secondary education (Table 1). Approximately one-third of the participants were married or cohabiting. A vast majority were taking medication for their depression, and over a third had been prescribed cardiovascular medication (i.e. antihypertensive or cholesterol-lowering). Nearly half of the participants were on long-term sick leave or a disability pension due to their depression. The mean CES-D score was 30·0 (sd 10·9), and the mean IDQ score was 8·4 (sd 2·0). Four participants had a CES-D score below the standard cut-off of 16 (FM arm n 2, BGM arm n 2). Close to a quarter of the participants already adhered to a healthy diet (IDQ ≥ 10). The participants in the control group rated their workability, compared to their lifetime best, lower on average (3·8 (2·2)) than participants in the intervention group (5·0 (1·8), P = 0·033).
Table 1. Baseline characteristics of all participants, participants in the FM intervention arm and BGM arm (Mean values and standard deviations; numbers and percentages; 95 % CI)
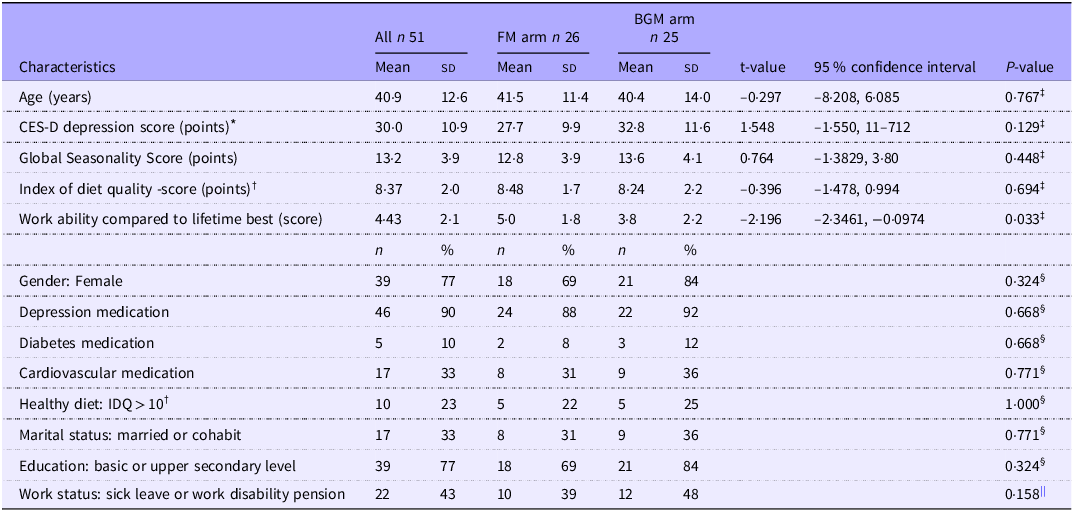
FM, Food for Mind; BGM, Bring Good Mood; CES-D, The Center for Epidemiologic Studies Depression Scale; IDQ, Index of Diet Quality.
* Intervention n 23, control n 20, total n 43.
† Intervention n 22, control n 20, total n 42.
‡ t test for between-group difference.
§ Fisher’s exact test for between-group difference.
|| Chi-squared test for between-group difference.
Feasibility
Reach and engagement
Participants were recruited through healthcare units to verify the eligibility and fulfilment of the diagnostic criteria. The recruitment started in February 2018 with one healthcare unit as a recruitment collaborator. By June 2021, the number of collaborators had increased to ten and seventy-six eligible participants had given their permission to pass on their contact details to the study. The total number of potentially eligible participants to whom the healthcare professionals introduced the study was impossible to determine. A registered dietitian was able to contact 88 % of the potentially eligible participants (Figure 1). About 16 % of those contacted declined, with reasons including insufficient mental resources (7·5 %), unsuitable timing of the group sessions (4·5 %), previous uncomfortable experience of group sessions (1·5 %), the expectation that participation would not be beneficial (1·5 %) or moving to another city (1·5 %).
Altogether, 76 % of those contacted were interviewed by a registered dietitian and gave their informed consent. Reasons for exclusion included participation in another nutrition intervention and the absence of an eligible diagnosis (Figure 1). It took an average of 6 months to recruit a sufficient number of eligible participants (8–12) who gave their informed consent to form one small group for both intervention arms. A total of fifty-one participants were randomised into the FM arm (n 26) and the BGM arm (n 25). Five small groups completed the intervention in both arms: January to March 2018, February to April 2019, January to March and October to December 2020 and March to May 2021. There were no differences in attendance in small group sessions between the arms. The mean attendance was 4·4 (sd 2·2) sessions in the FM small groups and 3·7 (sd 1·4) sessions in the BGM small groups. Nearly all participants in the FM arm and 60 % of those in the BGM arm attended at least one session. Only 23 % of the randomised participants in the FM arm and 16 % in the BGM arm participated in all six sessions (per protocol). Only one participant from the FM arm missed all the intervention sessions, compared to seven participants (28 %) from the BGM arm who were absent from all the sessions. Nearly two-thirds (63 %) of the randomised participants took part in the final evaluation at the end of the intervention. No adverse events were reported during either intervention.
Appropriateness and acceptability
Based on the questionnaire responses (FM arm n 13, BGM arm n 10) on whether the therapeutic conditions were established on a scale of 1–5, the participants in the FM arm felt that the content of the intervention met their needs and goals (mean 4·77, sem 0·122). They also felt understood and supported in the group sessions (mean 4·69, sem 0·133), felt active and engaged (mean 4·38, sem 0·213) and hopeful (mean 4·08, sem 0·211). The most significant differences compared with the BGM arm were found in the better alignment of the intervention content with needs and goals (P = 0·001), the success of the facilitator in maintaining effective group engagement (P = 0·010) and the participants’ feeling of hopefulness (P = 0·012; see Figure 3).
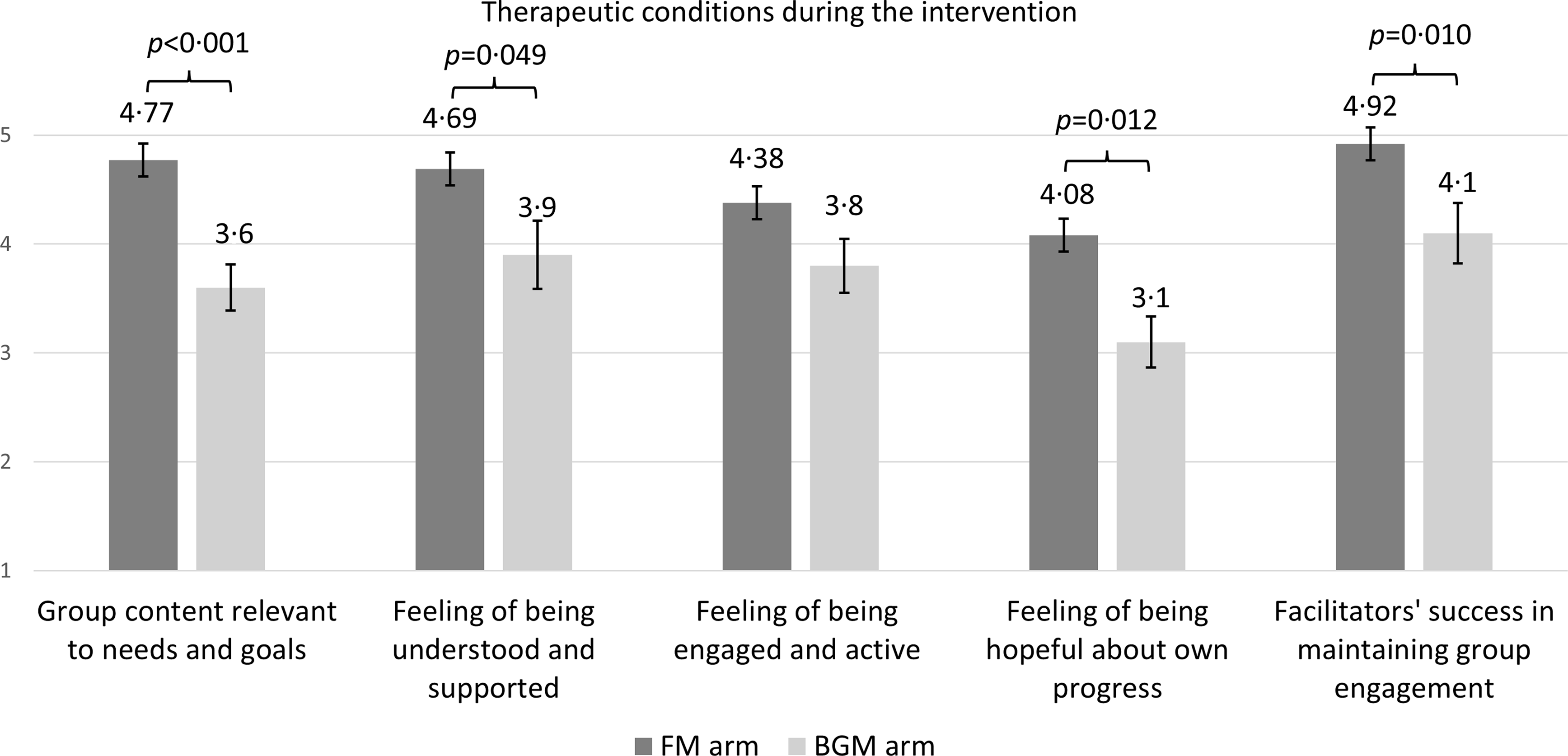
Figure 3. The established therapeutic conditions during the nutrition intervention (FM arm) and social support intervention (BGM arm). Data were available from a subgroup of participants (FM n 13, BGM n 8). Differences between means of intervention arms were analysed using the Mann–Whitney U test. Results are presented as means and standard error of the means (sem). FM, Food for Mind; BGM, Bring Good Mood.
Intervention acceptability among patients as the total TFA score was higher in the FM nutrition (n 13) intervention arm than in the BGM social support (n 10) intervention arm (mean 4·41 v. 3·66, P < 0·001). The largest and most significant differences in the TFA components between the FM arm and BGM arm were in the ‘perceived effectiveness’ component (mean 4·07 v. 3·03, P < 0·001) and ‘affective attitude’ component (how the individual feels about the intervention) (mean 4·85 v. 4·2, P = 0·008). The ‘intervention coherence’ component (the understandability of the intervention) was also higher in the FM intervention arm (mean 4·62 v. 3·9, P = 0·042) (Figure 4).
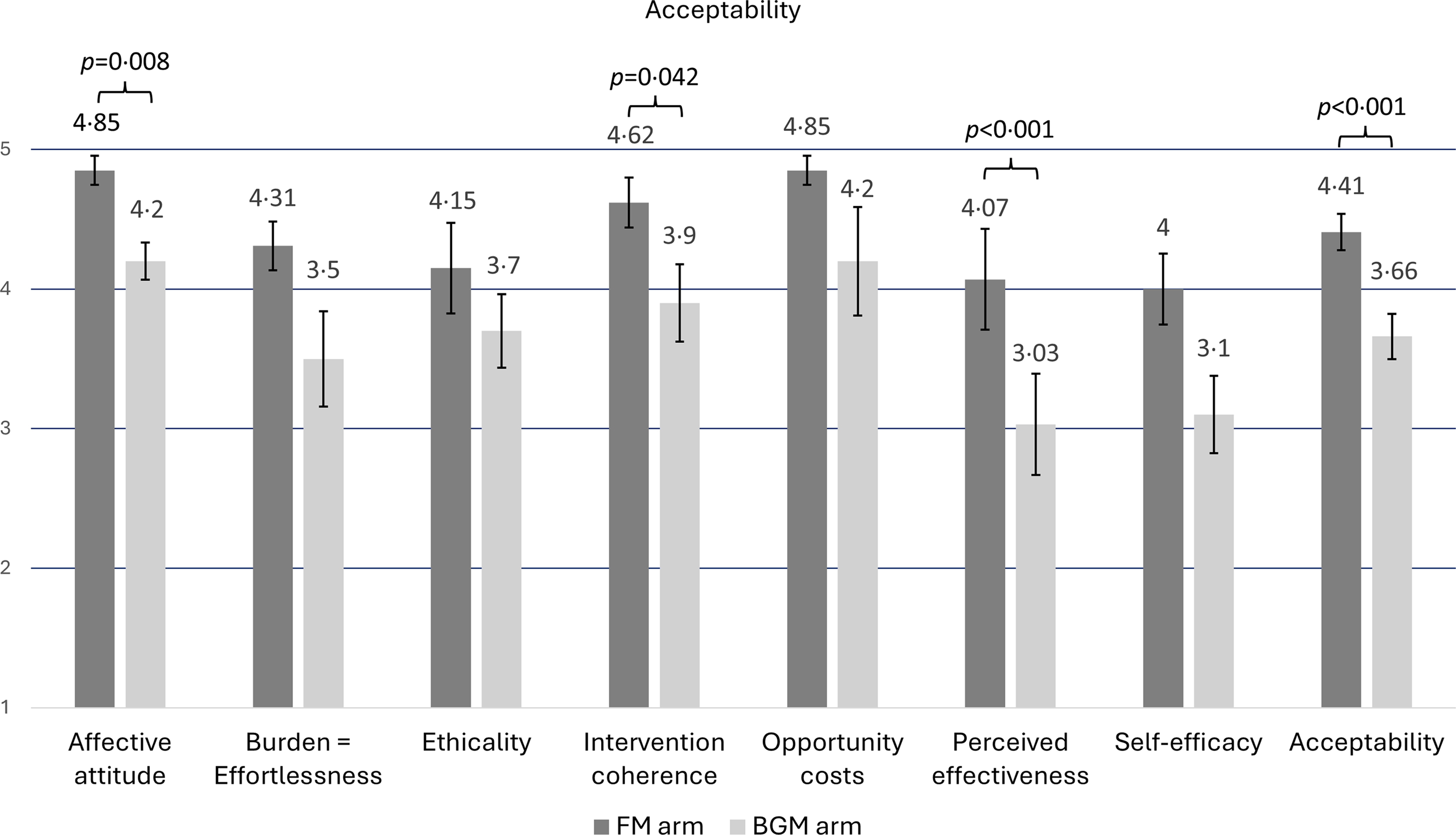
Figure 4. The acceptability of the nutrition intervention (FM arm) and social support intervention (BGM arm) based on Sekhon’s Theoretical framework of acceptability. Data were available from a subgroup of participants (FM n 13, BGM n 10). Total acceptability was calculated by summing up the scores of TFA components and dividing them by the number of components. Differences between FM and BGM arms were analysed using the Mann–Whitney U test. The results are presented as means and standard error of the means (sem). FM, Food for Mind; BGM, Bring Good Mood.
Short-Term effects
Diet quality
No notable differences in diet quality, as measured by IDQ, were observed between intervention arms or across time points (Table 2).
Table 2. The linear mixed effects model results (n 42), diet quality (IDQ) as a dependent variable (95 % CI)
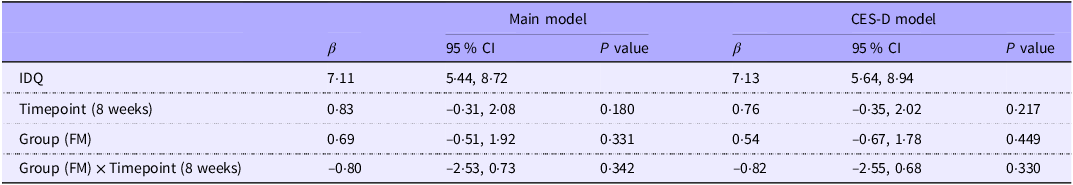
IDQ, Index of Diet Quality; FM, Food for Mind intervention group; CES-D The Center for Epidemiologic Studies Depression Scale.
CES-D model: adjusted for Main model and CES-D score.
Main model: adjusted for study group (FM/BGM), timepoint (baseline, 8 weeks), sex, age, depression medication use, treatment expectations (credibility and expectancy), Global Seasonality Score, marital status, educational level and work status.
Depressive symptoms
There were no statistically significant differences in depression symptom scores either between the groups or between the time points (Table 3). In the analysis, the GSS was positively associated (β = 3·77 (95 % CI: 1·42, 6·0), P = 0·009) and sex (male, β = –7·36 (95 % CI: −12·68, −2·11), P = 0·026) negatively associated with depressive symptom scores. These results indicate that higher seasonal variations in mood and behaviour were associated with increased depressive symptom severity, while the male sex was linked to lower depressive symptom scores.
Table 3. The linear mixed effects model results (n 42), depressive symptoms (CES-D) as a dependent variable (95 % CI)
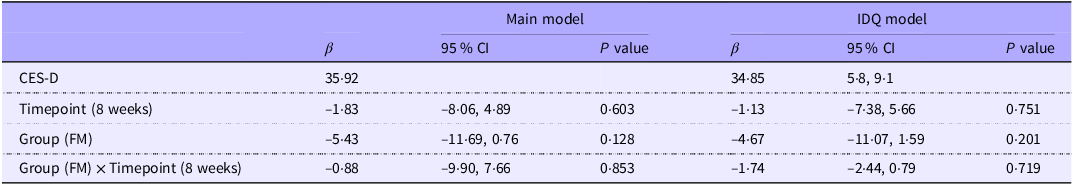
CES-D The Center for Epidemiologic Studies Depression Scale; IDQ, Index of Diet Quality; FM, Food for Mind intervention arm.
IDQ model: adjusted for Main model and IDQ score.
Main model: adjusted for study group (FM/BGM), timepoint (baseline, 8 weeks), sex, age, depression medication use, treatment expectations (credibility and expectancy), Global Seasonality Score, marital status, educational level and work status.
Discussion
To our knowledge, this pilot feasibility study is the first to examine the acceptability of a nutrition intervention in individuals with MDD. In addition to assessing the acceptability of the intervention and study procedure, we examined feasibility further based on reach, engagement and appropriateness. We also evaluated the short-term effect of the interventions on diet quality and depressive symptoms.
We were able to recruit and randomise fifty-one eligible participants into the FM and the BGM arms. Five small groups completed the intervention in both arms in the period 2018–2021. Adherence to the protocol, referring to attendance in all group sessions, was low: 23 % in the FM arm and 16 % in the BGM arm.
The FM arm was more successful in establishing therapeutic conditions than the BGM arm; the participants from the FM arm were more likely to feel understood, supported, engaged, active and hopeful in their progress in the intervention. They also found the content of the nutrition intervention relevant to their needs and goals.
The participants of the FM arm also considered the nutrition intervention with a strength-based counselling approach acceptable; they perceived the intervention as understandable, in line with their values and effective. They also found participation enjoyable, effortless and worth their time and effort. The ratings given by the FM arm participants were higher compared with the BGM arm ratings across these dimensions. These findings on participant-reported experiences are reassuring and speak for lifestyle interventions in mental health care, which was not self-evident due to a lack of previous research(Reference Jacka, O’Neil and Opie8–Reference Radkhah, Rasouli and Majnouni11).
Despite the participants’ perceptions of intervention effectiveness, we did not observe clinically or statistically significant within-group changes or between-group differences in changes in IDQ during the intervention. A slight decrease in depressive symptoms measured with CES-D was detected in both groups. Nevertheless, the changes within the groups and between-group differences were neither clinically nor statistically significant, which contradicts previous studies which detected improvements in diet quality and depressive symptoms(Reference Jacka, O’Neil and Opie8–Reference Radkhah, Rasouli and Majnouni11). In this study, the mean IDQ score (±sd) of the clinically depressed people at baseline was around 8·4 (2·0), and nearly a quarter (23 %) of the participants had a healthy diet (IDQ score of 10 or above)(Reference Leppälä, Lagström and Kaljonen31). This may have affected the group’s means and influenced the level of diet quality in the groups. The mean CES-D score at baseline was 30·0, indicating relatively severe depressive symptoms; the score varied from 4 to 50, with four participants scoring below the standard cut-off score of 16. As this study aimed to examine the feasibility of interventions, we strived to include a diverse patient population, which is typical for a study conducted in a real-world setting(Reference Skivington, Matthews and Simpson12). Therefore, we also included some participants with a baseline high-quality diet (IDQ ≥ 10) or a CES-D score below the standard cut-off score of 16 in the study. This differs from previous RCT, which included only patients with poor diet quality(Reference Jacka, O’Neil and Opie8–Reference Bayes, Schloss and Sibbritt10) and the level of moderate or severe depressive symptoms at baseline(Reference Jacka, O’Neil and Opie8,Reference Parletta, Zarnowiecki and Cho9) . However, Radkhal et al. also used the CES-D(Reference Radkhah, Rasouli and Majnouni11) and, similar to our study, found no clinically significant reduction in depressive symptoms. The effectiveness of the intervention was not the primary outcome of our study. Nevertheless, both lower baseline diet quality and current moderate or severe depressive symptoms assessed with a depressive symptom scale, in addition to the diagnosis of MDD, should be considered as an inclusion criterion in clinical trials investigating the effectiveness of an intervention. This aligns with the recently published methodological and reporting guidelines for clinical trials in nutritional psychiatry as of 2024(Reference Marx, Visser and Wallace44). Furthermore, we did not consider power calculations in this pilot trial because the primary objective was to assess the feasibility of the study design, procedures and interventions rather than clinical effectiveness. The focus was on recruitment, participant adherence, retention, data collection methods and acceptability evaluations. The decision was based on practical considerations and pilot study guidelines(Reference Teresi, Yu and Stewart45).
Strengths and limitations
To the best of our knowledge, this pilot feasibility study is the first RCT to study the acceptability of a nutrition intervention in people with MDD alongside a feasibility assessment. Evaluating the feasibility and acceptability is recommended before designing and implementing a large-scale intervention study(Reference Skivington, Matthews and Simpson12). The participants were recruited in collaboration with several healthcare services to ensure diagnostic eligibility. Also, considerable effort was devoted to designing a nutrition intervention with a theory-based approach used throughout the intervention consisting of sessions, counselling, activities and home assignments. In addition, a registered dietitian with experience in group counselling facilitated the strength-based counselling of the nutrition intervention. Furthermore, the study controlled the possible confounding effect of peer support with a comparison group (BGM arm), which followed a befriending protocol widely used in psychological interventions(Reference Bendall, Jackson and Killackey27). Each FM and BGM intervention small group was also designated a closed social media (WhatsApp) group for peer support during the intervention. The acceptability of the intervention was evaluated with a questionnaire developed based on Sekhon’s multi-construct theoretical framework of acceptability(Reference Sekhon, Cartwright and Francis30), and the short-term effects of the intervention were measured with validated questionnaires. The assessment tool for diet quality (The Index of Diet Quality) fits well into the Finnish food culture, and it is validated in Finland(Reference Leppälä, Lagström and Kaljonen31). Depressive symptoms were measured with a widely used CES-D questionnaire(Reference Mäkelä32).
However, several limitations should also be acknowledged. Even though depression rates are relatively high(Reference Koponen, Borodulin and Lundqvist46) and mental health services struggle to meet the growing numbers of patients(Reference Lampinen and Lilja47) in Finland, the study faced considerable challenges in participant recruitment and retention. Despite several face-to-face presentations concerning the research and the recruitment procedures to the healthcare staff involved in recruitment and information about the FM study produced and distributed for healthcare workers to help them introduce the study to patients, a need to convince the staff persisted. The COVID-19 pandemic significantly affected recruitment, which was operationally inactive due to a six-month lockdown. After pandemic-related live group restrictions had been relaxed, restarting recruitment in healthcare organisations was still challenging due to a backlog in treatment caused by the pandemic, and the recruitment pace remained slow.
The decision to conduct recruitment in collaboration with healthcare organisations as a part of their daily routines can be considered a major limitation of our study. With this approach, we were unable to monitor and record the total number of potential participants declining after the study was introduced by the healthcare professionals and the reasons behind the refusals. This limitation highlights a broader issue, at least within the Finnish healthcare system, where the routine monitoring of patients’ referral pathways to different treatments is insufficient. As a result, healthcare organisations lack adequate data on how effective different interventions are in reaching their target populations. Addressing this gap is necessary to enhance treatment delivery and ensure that treatments reach their target populations.
Engaging participants to attend the group sessions according to the protocol proved difficult, resulting in only a minority adhering to all intervention sessions. This might influence the effectiveness outcomes and should be considered when performing power calculations. Fortunately, there were no ongoing intervention groups during the pandemic. Considerable effort was made to enhance retention throughout the intervention, including reminder emails and social media (WhatsApp) messages before each group session. However, even after the pandemic-related live group restrictions had been relaxed, many people still felt nervous and tried to avoid face-to-face meetings with other people. This may also have been the case with the participants in this study. It should also be noted that, in general, reaching and engaging people with mental illnesses in clinical trials has been difficult(48), and various strategies and methods have been developed and utilised to improve recruitment. Recruitment strategies that rely on healthcare professionals’ indications might face challenges, e.g. in providing key information about the study due to lack of time or a lapse in memory, misconceptions, or misunderstanding of the differences between the intervention arms or the role of the randomisation process(Reference Howard, de Salis and Tomlin49), all of which may lead to suboptimal recruitment results.
In addition, in previous research, increased costs have been reported as one of the main factors challenging adherence to the recommended diet(Reference Radkhah, Rasouli and Majnouni11,Reference Bayes, Schloss and Sibbritt18) . Over 40 % of our study participants were either on long-term sick leave or on a disability pension due to depression, which may have caused financial challenges for participation in the group sessions or the study visits, as travelling expenses were not reimbursed. Several studies have acknowledged this by offering food hampers to participants(Reference Jacka, O’Neil and Opie8–Reference Bayes, Schloss and Sibbritt10). However, it has also been shown that adhering to the recommended intervention diet (modified Mediterranean diet) was more affordable compared to the participants’ typical diet(Reference Chatterton, Mihalopoulos and O’Neil50). Still, getting people with MDD to participate in clinical interventions requires substantial effort. Engaging people with MDD and recruiting (healthcare) staff already in the design phase of the study might improve recruitment by finding targeted recruitment strategies and methods.
Getting responses to the study questionnaires was another challenge. Questionnaires were offered electronically and on paper to increase response rates, and multiple reminders were sent. The pandemic affected response rates at the end of the intervention assessments for one FM and one BGM small group. The questionnaire included many other measures alongside those reported here, which might have also resulted in an excessive response burden for people with moderate or severe MDD and influenced the response rates and the amount of missing data in many measures.
Acceptability and appropriateness (whether the therapeutic conditions were established during the intervention) were measured at the end of the final FM and BGM intervention sessions and were available from a subset of participants, so caution must be exercised regarding the generalisability of these findings. In addition, despite the high total acceptability rate of the FM intervention arm and statistical differences in the components of acceptability compared to the BGM arm, the result of the self-efficacy component, examined with the statement ‘I’ve been able to introduce these lifestyle choices into my everyday life’, had the lowest score. This may indicate that even though the core of the nutrition intervention was to make small and realistic dietary changes at the time, 8 weeks could still be too short of a time to introduce lifestyle changes into the daily routines of people with clinical depression. Another explanation could relate to the nutrition intervention’s lack of practicality and adaptability to everyday life. This underlines the need for developing and refining interventions together with the individuals with lived experience (in this case, people with MDD) and those involved in intervention delivery and implementation, which might result in increased uptake and engagement with the intervention, thus enhancing the effectiveness of the intervention(Reference Skivington, Matthews and Simpson12). Furthermore, in this study, acceptability was only assessed from the intervention participants’ point of view, whereas for successful implementation, acceptability from the deliverers’ perspective is equally important(Reference Sekhon, Cartwright and Francis14).
Even though the IDQ questionnaire has been developed in Finland and reflects adherence to the Nordic dietary recommendations(Reference Becker, Lyhne and Pedersen51), we discovered its limitations in assessing the diet quality of people with MDD. The capability of the IDQ to detect minor changes as an indexing tool might be limited due to a reasonably strict scoring scale. In addition, an 8-week intervention might be too short to achieve changes in diet that are detectable with the tool used in this study. Furthermore, the 8-week period might also be inadequate to achieve a substantial nutritional change or a significant improvement that could influence depressive symptoms. Previous studies have demonstrated the positive effects of 12-week interventions(Reference Jacka, O’Neil and Opie8–Reference Bayes, Schloss and Sibbritt10).
Finally, the difference between the arms in how the closed social media (WhatsApp) groups were used during the intervention can be considered a limitation. Furthermore, data on the frequency of the use of WhatsApp groups was not available, resulting in an inability to assess any differences in the intensity of the interventions or the independent effects of peer groups. However, activity in these groups was relatively low at best, and the impact on intervention intensity and effect was likely negligible.
In conclusion, this pilot study aimed to assess the feasibility through the reach and engagement of the participants, the appropriateness and acceptability of intervention procedures among the participants, as well as the short-term effect of the interventions on diet quality and depressive symptoms. The nutrition intervention based on behaviour change theories and related techniques was found acceptable as an adjunctive treatment for people with MDD, and its acceptability was significantly higher compared to the social support intervention. However, the challenges in recruiting and engaging people with MDD in the interventions and study procedures exceeded our initial expectations. In future research, co-designing the interventions and study procedures with people with MDD, the parties implementing the intervention, and other stakeholders, starting in an early phase of the intervention development process, is recommended. Targeted recruitment strategies are also needed to reach and engage people with MDD to participate in clinical interventions. In addition, there is a need to consider combining new approaches, such as digital solutions, for delivering interventions based on the behaviour change theory to increase engagement with the interventions and foster lifestyle changes.
Acknowledgements
We sincerely thank the healthcare units from the public sector (Kuopion Psykiatrinen Keskus, Siilinjärven Mielenvireyspalvelut and Kuopion Kaupungin Mielenterveyspalvelut), private sector (Savon Psykiatripalvelu, Lääkärikeskus Aava, Mehiläinen, Pihlajalinna, Terveystalo), occupational healthcare (Järviseudun työterveys) and the Finnish Student Health Service in the Northern Savo area for collaboration during this research. We extend special thanks to all the organisations’ healthcare workers for their time and input in recruitment. We would also like to thank the non-profit organisation Turvalinkki for facilitating the small groups in the social support arm. This research would not have been possible without the participants, to whom we are deeply grateful. Additionally, we would like to thank Petrus Nuotio for his assistance in statistical analyses and Elisa Wulff for reviewing and refining the language of this manuscript.
This study was supported by the Social Insurance Institution of Finland (grant number 35/26/20), Jenny and Antti Wihuri Foundation (J.R., grant number 220189 N1), Emil Aaltonen Foundation (J.R., grant number 00210306) and Yrjö Jahnsson Foundation (J.R., grant number 20237697). Social Insurance Institution of Finland, Jenny and Antti Wihuri Foundation, Emil Aaltonen Foundation, and Yrjö Jahnsson Foundation had no role in the design of the study, analysis of the results or writing of this article.
J. R. carried out the FM study during 2019–2021 as a research dietitian, contributed to funding acquisition, formulating research questions, curation and formal analyses of the data, and writing the original draft. A. R. contributed to project administration and reviewing and editing the article. O. N., T. P. and P. A. contributed in conseptualisation and designing the study, methodology, formulating the research questions and reviewing and editing the article. O. N. contributed in project administration and funding acquisition. A. R., O. N., T. P., and P. A. contributed to supervision.
The authors declare no conflicts of interest.
This RCT was conducted according to the guidelines in the Declaration of Helsinki, and all study procedures were reviewed by the Northern Savo Hospital District Committee on Research Ethics (409/2017). Written informed consent was obtained from all participants. This study is registered with the USA National Library of Medicine ClinicalTrials.gov. (NCT03904771, https://clinicaltrials.gov/study/NCT03904771).









