Introduction
The flight phenology of Afrotropical ants is little known. Light-trap studies have been reported for several African Dorylinae in Uganda (Bowden and Church, Reference Bowden and Church1973; Bowden and Morris, Reference Bowden and Morris1975; Haddow et al., Reference Haddow, Yarrow, Lancaster and Corbet1966) and in Ghana (Leston, Reference Leston1979). Also in Ghana, Gibbs and Leston (Reference Gibbs and Leston1970) trapped gynes of the formicine Oecophylla longinoda (Latreille) and the ponerine Odontomachus troglodytes Santschi (as Od. haematodus Auctt., a misidentification corrected by Brown, Reference Brown1976). Lenoir and Dejean (Reference Lenoir and Dejean1994) trapped gynes of formicines Polyrhachis militaris Fabricius and P. laboriosa (F. Smith) in Cameroon.
Nene et al. (Reference Nene, Rwegasira, Nielsen, Mwatawala and Offenberg2016) observed nuptial flights of Oe. longinoda during the rainy season in Tanzania, confirming observations of Way (Reference Way1954) and Rwegasira et al. (Reference Rwegasira, Mwatawala, Rwegasira and Offenberg2015) from nest examinations in the same region. Marchart (Reference Marchart1972) reported gynes of Oe. longinoda in nests every month of the year in Ghana, with peak numbers in January–March and June–September. Although diurnal nuptial flights of some African Ponerinae have been reported (Villet et al., Reference Villet, Crewe and Robertson1989), there are no comprehensive survey reports of diurnal flight activity among Afrotropical ants. In Brazil, in one study, 95% of ant species flew diurnally (Feitosa et al., Reference Feitosa, da Silva and Aguiar2016), whereas in Puerto Rico at two contrasting sites, 66% and 78% of ant species flew nocturnally (Torres et al., Reference Torres, Snelling and Canals2001).
By contrast to the limited information on the flight phenology of Afrotropical ants that for the Neotropical ant fauna has received greater attention (Cantone, Reference Cantone2018; Donoso et al., Reference Donoso, Basset, Shik, Forrister, Uquillas, Salazar-Mendez, Arizala, Polanco, Beckett, Diego Dominguez and Barrios2022; Kannowski, Reference Kannowski1969, Reference Kannowski1991; Kaspari et al., Reference Kaspari, Pickering, Longino and Windsor2001a, Reference Kaspari, Pickering, Longino and Windsorb; Nascimento et al., Reference Nascimento, Delabie, Ferreira and Della Lucia2004, Reference Nascimento, Delabie and Della Lucia2011; Ortius and Lechner, Reference Ortius and Lechner1997; Rettenmeyer, Reference Rettenmeyer1963; Schneirla, Reference Schneirla1948, Reference Schneirla1972; Torres et al., Reference Torres, Snelling and Canals2001; Tozetto et al., Reference Tozetto, Forrister, Duval, Hays, Garwood, Vargas Castro, Lattke, Sendoya and Longino2023; Uquillas et al., Reference Uquillas, Bonilla, Arizala, Basset, Barrios and Donoso2025), augmented by similar studies of several of the same species in the southern Nearctic and Caribbean bioregions (Baldridge et al., Reference Baldridge, Rettenmeyer and Watkins1980; Cantone, Reference Cantone2018; Deyrup and Trager, Reference Deyrup and Trager1986; Hart and Tschinkel, Reference Hart and Tschinkel2012; Kusnezov, Reference Kusnezov1962).
In order for genetic mixing to occur, individual colonies of conspecific ant species must coordinate their flights. The main meteorological factors found to influence ant nuptial flights are rainfall and temperature (Hölldobler and Wilson, Reference Hölldobler and Wilson1990). It has been argued that rainfall is the more important factor in tropical regions where there is low significant variation in temperatures (Brown, Reference Brown, Meggers, Ayensu and Duckworth1973; Kaspari et al., Reference Kaspari, Pickering, Longino and Windsor2001a; Torres et al., Reference Torres, Snelling and Canals2001). However, in a 1-year study of 15 doryline species in tropical Brazil, temperature was the only significant meteorological factor affecting nightly flight phenology (Nascimento et al., Reference Nascimento, Delabie and Della Lucia2011), and similarly for the diurnal flying Myrmicinae Atta vollenweideri Forel in sub-tropical Argentina (Staab and Kleineidam, Reference Staab and Kleineidam2014). Temperature was an important driver for nuptial ant flights in Barro Colorado Island (BCI), Panama (Donoso et al., Reference Donoso, Basset, Shik, Forrister, Uquillas, Salazar-Mendez, Arizala, Polanco, Beckett, Diego Dominguez and Barrios2022; Uquillas et al., Reference Uquillas, Bonilla, Arizala, Basset, Barrios and Donoso2025) and for Dorylinae at sites in Brazil, Costa Rica and Ecuador (Tozetto et al., Reference Tozetto, Forrister, Duval, Hays, Garwood, Vargas Castro, Lattke, Sendoya and Longino2023). However, Nene et al. (Reference Nene, Rwegasira, Nielsen, Mwatawala and Offenberg2016) reported that although Oe. longinoda flew mainly at dusk in the rainy season in Tanzania; rainfall and temperature were unimportant as flight stimulants.
We present data on the phenology of nocturnally flying Afrotropical ants from 42 months of nightly light-trapping. Our aims were to determine the periodicity and seasonality of nocturnal mating flights and to assess whether rainfall and monthly temperature influenced flight activity.
Materials and methods
A Rothamsted light-trap (Williams, Reference Williams1948) fitted with a 150-watt clear incandescent filament bulb was operated nightly from 10 October 1974 to 31 March 1978, a total of 1258 nights. Owing to power failures the trap was inoperative on 11 nights (20–23 March, 8–9 July 1975; 27 January, 16 September, 24 October 1976; 18 January, 28 October 1977). The trap was illuminated from 18:00 to 06:00 h. The collecting bottle contained absorbent paper impregnated with dichlorvos (2,2-dichlorovinyl dimethyl phosphate) as a killing agent. The trap was sited in open ground 1.2 m above mown grass in the residential area of the Cocoa Research Institute Ghana (CRIG), Tafo, at least 20 m from the nearest buildings. The surrounding area comprised cacao plots under thinned secondary forest tree shade, open areas, other residential compounds and patches of farm-bush and secondary forest.
Winged ants were identified and sexed using a Wild stereo-zoom microscope. Representative specimens of unidentified taxa were card-mounted, coded uniquely and used for reference thereafter. Owing to the lack of systematic descriptions of alate ants, specimens were compared, and identified where possible, with named associations between castes in the CRIG’s museum collection. In the absence of matched named examples, gynes and males were linked from morphological similarities and wing venation patterns. Cantone (Reference Cantone2019) has since published dichotomous keys to identify ant genera worldwide based initially on wing venation. Where males have not been collected previously with workers or gynes the species affiliation is marked cf. to emphasise some uncertainty in specific designation. The identities of some taxa were determined by Barry Bolton (NHMUK), with whom representative specimens were deposited. All ant names have been updated to conform to those accepted currently (Anon, 2024), and their authorities are listed in table 1.
Table 1. Species/morphospecies and abundance of Formicidae collected in a light-trap at Tafo, Ghana, from October 1974 to March 1978
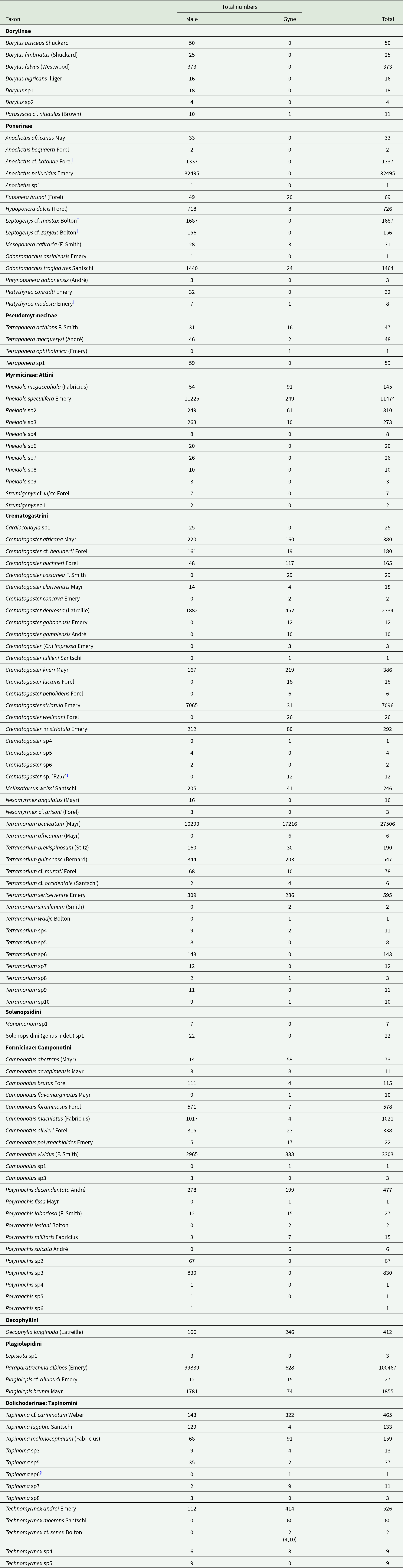
† Barry Bolton (pers. comm.) determined the species as A. cf. punctatus Santschi, now a junior synonym of A. katonae (Brown, Reference Brown1978).
¡As listed in the CRIG collection.
‡ Determined by Barry Bolton.
§ Gyne with eight antennal segments as speculated by Shattuck (Reference Shattuck1992, p. 149).
Data analysis
Data were analysed using GenStat9 (Payne et al., Reference Payne, Harding, Murray, Soutar, Baird, Welham, Kane, Gilmour, Thompson and Webster2006). Kaspari et al.’s (Reference Kaspari, Pickering, Longino and Windsor2001a) definition of common species as those with individuals trapped 20+ times was adopted. Individuals of 65 species/morphospecies were trapped between 20 and 100467 times. In order to assess the overall peak months of flight activity for common species/morphospecies and their relations to seasonal rainfall patterns, the proportions trapped each calendar month (corrected for the irregular numbers of months trapped by multiplying January–March and October–December counts by 0.75) were calculated and then ranked incrementally from no flights (zero) to the month with highest proportions and compared by ANOVA.
In order to assess whether rain affected captures, rainfall data were coded as dates with (1) or without rain (0), and similarly for ant capture (1) and non-capture dates (0), starting from the first date of capture of each species/morphospecies. The resultant sums in the four categories were compared using 2 × 2 contingency tests. Yates (Reference Yates1934) correction was applied when any cell had fewer than ten individuals. The influence of rain, temperature, and their interaction on monthly catches was assessed by linear regression. In order to stabilise variances, the monthly catch (n) was transformed Log10 (n + 1), as too was total monthly rain to Log10 (mm + 1) in order to incorporate data for January 1975 which, uniquely in the present study, was rainless. Temperatures are usually lowest at night, so the mean monthly minimum temperatures were chosen, as these are most likely to have influenced ants’ nocturnal flight activities. Catch data are presented as twelve month plots of proportions of totals caught over the 42 month trapping period, corrected as above for the unequal months of trapping, and similarly for the proportion of the total rainfall in each month.
Rainfall and temperature data were obtained from the CRIG’s weather station situated 0.7 km from the trap site.
Results
The climate at the CRIG (6.22° N, 0.37° W), situated in the moist semi-deciduous tropical forest zone, is equatorial with two rainy seasons annually (Walker, Reference Walker and Wills1962). The main and minor rains (usually March–June and September–November, respectively) are separated by a short dry season around August, which is also associated with lowest maximum temperatures (fig. 1). A longer dry period occurs from December–February, during which night time temperatures are at their lowest. However, some rain is likely in all months. Mean annual rainfall is above 1600 mm (Walker, Reference Walker and Wills1962). In the 42-month study period, January 1975 was the only month without rain (fig. 1). Mean daily temperatures varied little throughout the study period at 25.9 ± 1.3°C (±SD). Mean minimum temperatures were more variable at 21.0 ± 1.7°C (±SD), with overnight temperatures dipping in March, and an absolute overnight low of 11.1°C on 27 January 1976.
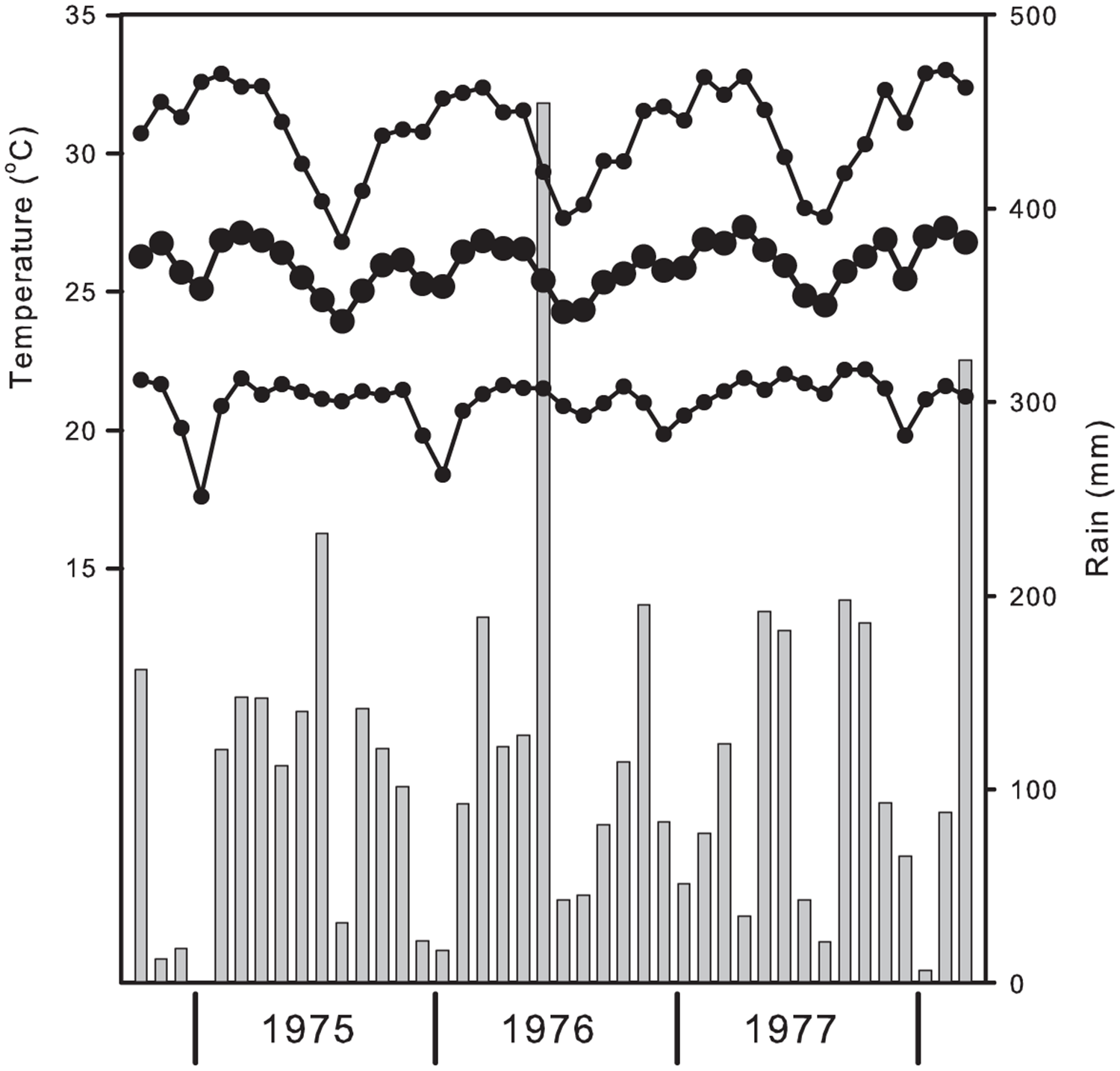
Figure 1. Mean monthly maximum, average and minimum temperatures and monthly rain (histogram).
A total of 204393 ants was collected (table 1). Sub-families comprised: Dorylinae, 497 individuals, 99.8% male, two genera; Pseudomyrmecinae, 155 individuals, 93.2% male, one genus; Ponerinae, 39942 individuals, 99.9% male including 1897 males of 13 species/morphospecies from unidentified genera, >16 genera; Myrmicinae, 52704 individuals, 63.2% male including 22 males of Tribe Solenopsidini (genus indet.) sp1, nine genera; Formicinae, 109667 individuals, 98.5% male, six genera; Dolichoderinae, 1428 individuals, 36.0% male and two genera. Although Myrmicinae was the most speciose sub-family, the highest catch was of the Formicinae Paraparatrechina albipes which comprised 42.2% of the ants caught. Of the 135 species/morphospecies collected, 40 could not be identified beyond genus, and males of a further 14 beyond subfamily, however, that 40% of species/morphospecies comprised <2% of the total number of ants caught.
Our ant capture and daily weather records are available (Supplementary material file S1). Flight dates are extracted for all named and tentatively named species/morphospecies not represented in figs. 3–6 (Supplementary material file S2). Species/morphospecies accumulation and total ant abundance over time are presented in Supplementary material file S3.
As regards the total numbers of ants caught in each sub-family; Dorylinae showed their strongest correlation with peak rainfall in June (fig. 2). Ponerinae, Pseudomyrmecinae and Myrmicinae were trapped most frequently at the middle of the main rains, while Dolichoderinae were mostly caught in wet months, but with numbers that increased rapidly from their lows in the driest months of December–January and August. The numbers of Dolichoderinae caught accelerated from the start of the main rains in February, then remained high for the next three wet months before declining in the wet months of June and July, only to increase again at the start of the minor rains in September. Numbers of Dolichoderinae remained high in the wet months of October and November. Numbers of P. albipes trapped were ca. 10x higher than all other Formicinae combined. That species is analysed separately so as not to swamp the collective responses of other species in that sub-family. Catches of Formicinae (less P. albipes) increased to their maximum in March, the first month of the main rains, whereas P. albipes numbers peaked in February, with a smaller peak at the start of the minor rains in September, following lows in the driest months of December–January and August.
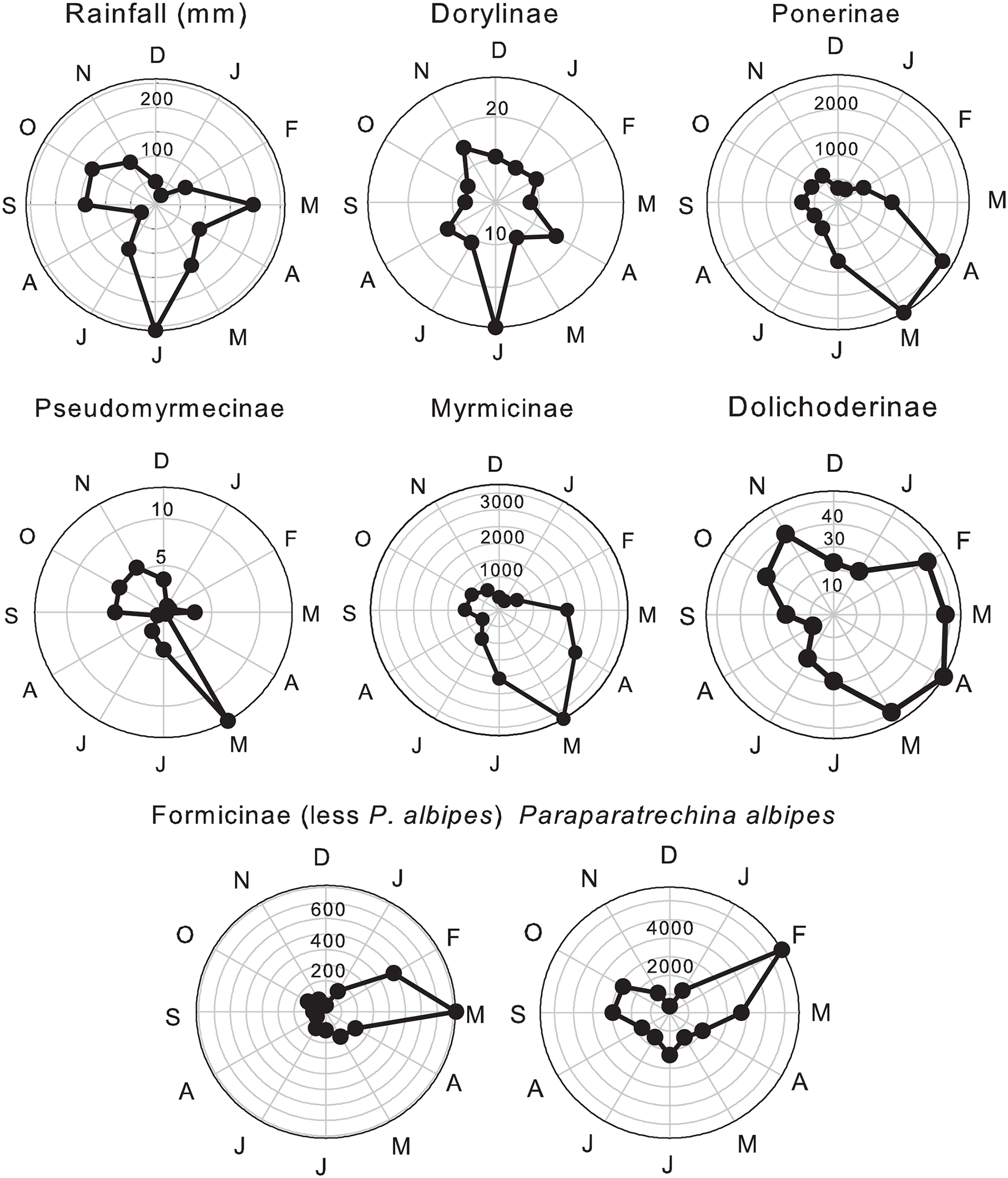
Figure 2. Mean monthly rainfall and mean monthly catches of total winged ants in six subfamilies with Paraparatrechina albipes treated separately from all other Formicinae.
Common species/morphospecies flew during a mean 10.1 ± 0.36 (SEM) months, with no significant differences between sub-families (mean ranges: Formicinae 9.1–11.3 Dolichoderinae). Hence the majority of species/morphospecies in all sub-families were trapped throughout the year, but with taxa-specific variation in their monthly abundance. Peak flight activity for common species coincided with the onset of the main rains (February–March) with a smaller secondary peak associated with the onset of the minor rains (September–October) and troughs in the drier months of January and August (figs. 3–6, fig 3b F 11, 732 = 11.02, P < 0.001).
Dorylus atriceps (fig. 3), Platythyrea conradti (fig. 4), Cardiocondyla sp., Crematogaster nr striatula males (fig. 5), Plagiolepis brunni gynes (fig. 6) and Plat. modesta, Technomyrmex sp4 and sp5 and Cr. [F257] gynes (table 1) were the only species/morphospecies whose peak flight activity coincided with the December–January dry season. The peak flights of all other species/morphospecies coincided with months in one or both rainy seasons (figs. 3–6) and, with the exceptions of Tetramorium guineense males, Tet. cf. muralti (fig. 5), Tet. sp4 (table 1) and Tech. moerens gynes (fig. 4), higher in the main than in the minor rains. Polyrhachis militaris and Tapinoma sp3 (table 1) also exhibit minor rainy season peaks, but the data for these species/morphospecies are based on captures of 15 and 13 individuals, respectively.
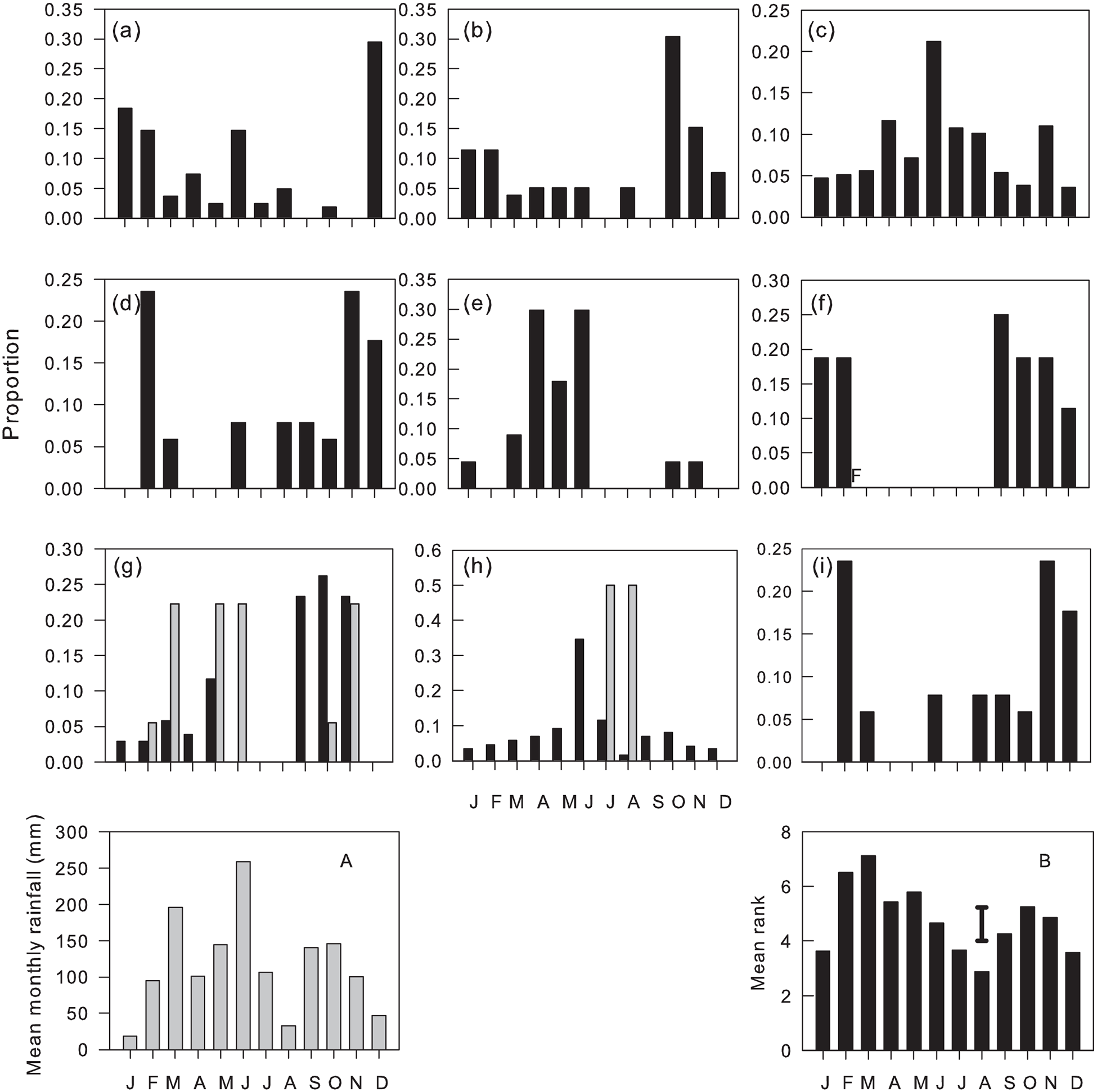
Figure 3. Monthly proportions of flights of Dorylinae: (a) Dorylus atriceps, (b) D. fimbriatus, (c) D. fulvus, (d) D. nigricans, (e) Dorylus sp4, (f) Parasyscia nitidulus and Pseudomyrmecinae (g) Tetraponera aethiops, (h) T. mocquerysi, and (i) Tetraponera sp1. Males (black columns), gynes (grey columns and inserted ‘F’ for P. nitidulus). (A) Monthly rainfall. (B) Mean rank of proportions for common species/morphospecies flying each month (vertical bar = LSD05).
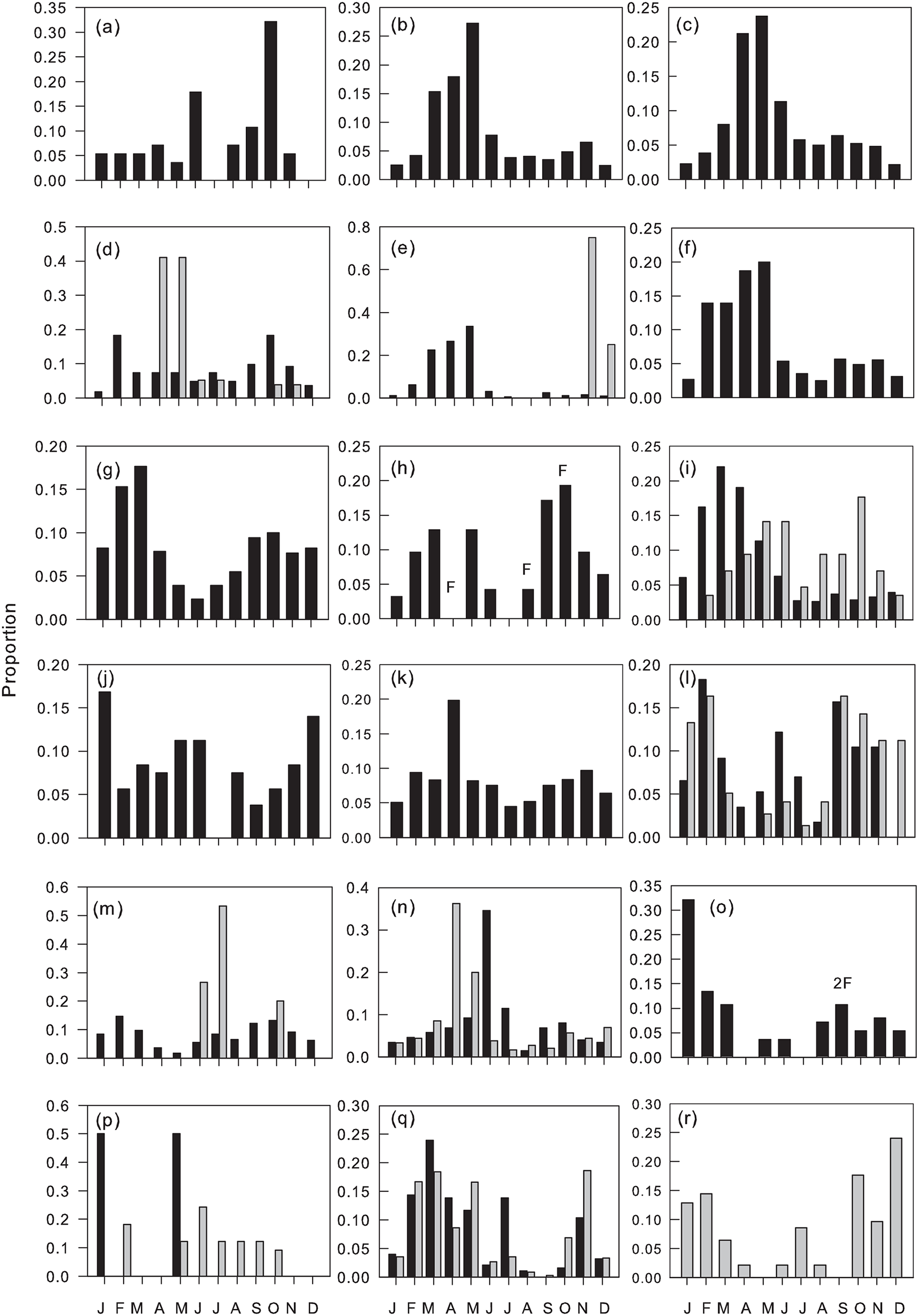
Figure 4. Monthly proportions of flights of Ponerinae: (a) Anochetus cf. africanus, (b) A. cf. katonae, (c) A. pellucidus, (d) Euponera brunoi (Forel), (e) Hypoponera dulcis, (f) Leptomastix cf. mastax, (g) L. cf. zapyxis Bolton, (h) Mesoponera cf. caffraria, (i) Odontomachus troglodytes, (j) Platythyrea conradti, (k) Ponerinae (genus indet.) sp1 and Dolichoderinae, (l) Tapinoma cf. carininotum Weber, (m) T. lugubre Santschi, (n) T. melanocephalum, (o) Tapinoma sp5, (p) Tapinoma sp7, (q) Technomyrmex andrei, (r) T. moerens. Males (black columns), gynes (grey columns and inserted ‘F’).
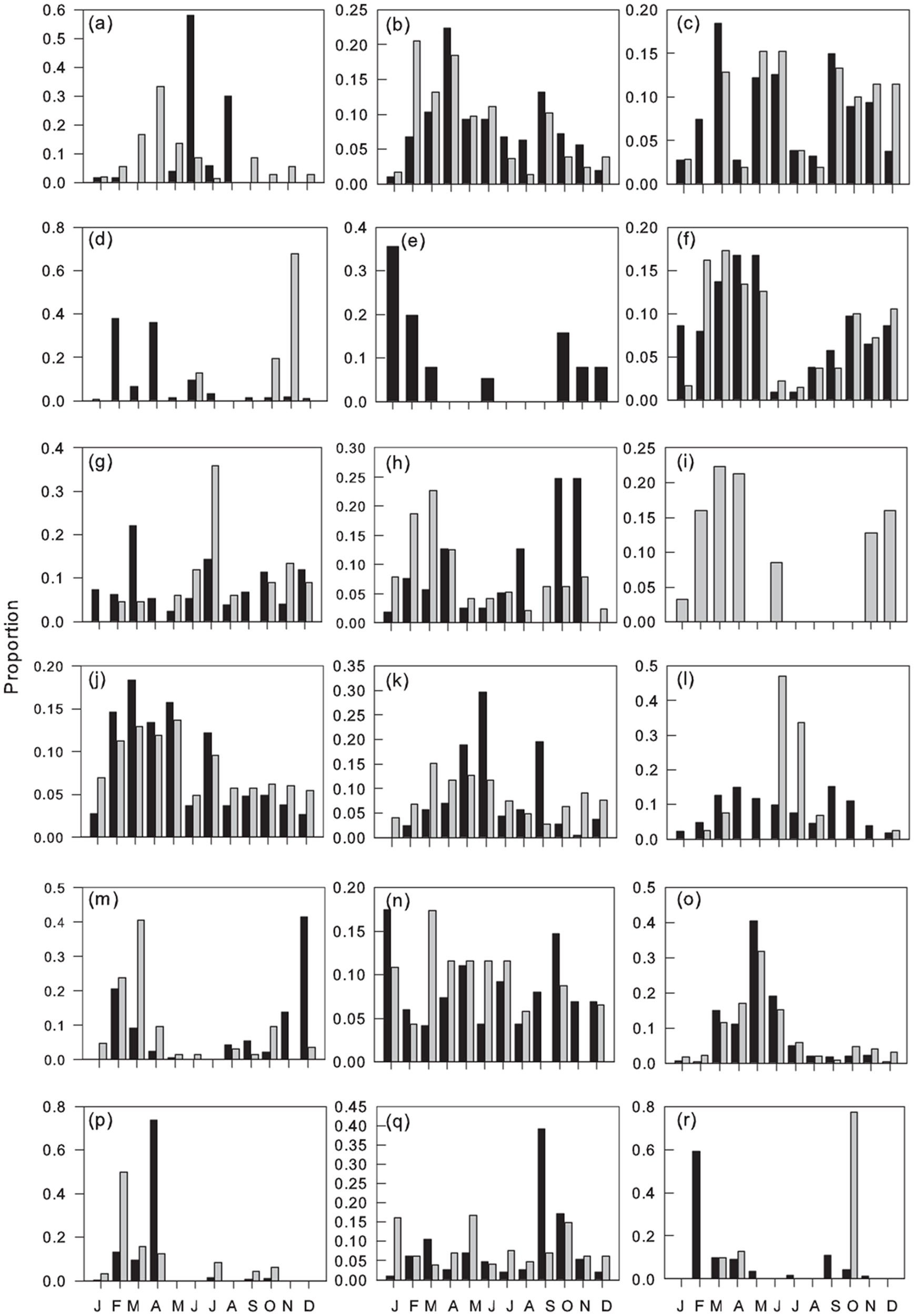
Figure 5. Monthly proportions of flights of Myrmicinae: (a) Pheidole megacephala, (b) P. speculifera, (c) Pheidole sp2, (d) Pheidole sp3, (e) Cardiocondyla sp., (f) Crematogaster africana, (g) C. cf. bequaerti, (h) C. buchneri, (i) C. castanea, (j) C. depressa, (k) C. kneri, (l) C. striatula, (m) C. nr striatula, (n) Melissotarsus weissi, (o) Tetramorium aculeatum, (p) T. brevispinosus, (q) T. guineense, (r) T. cf. muralti. Males (black columns), gynes (grey columns).
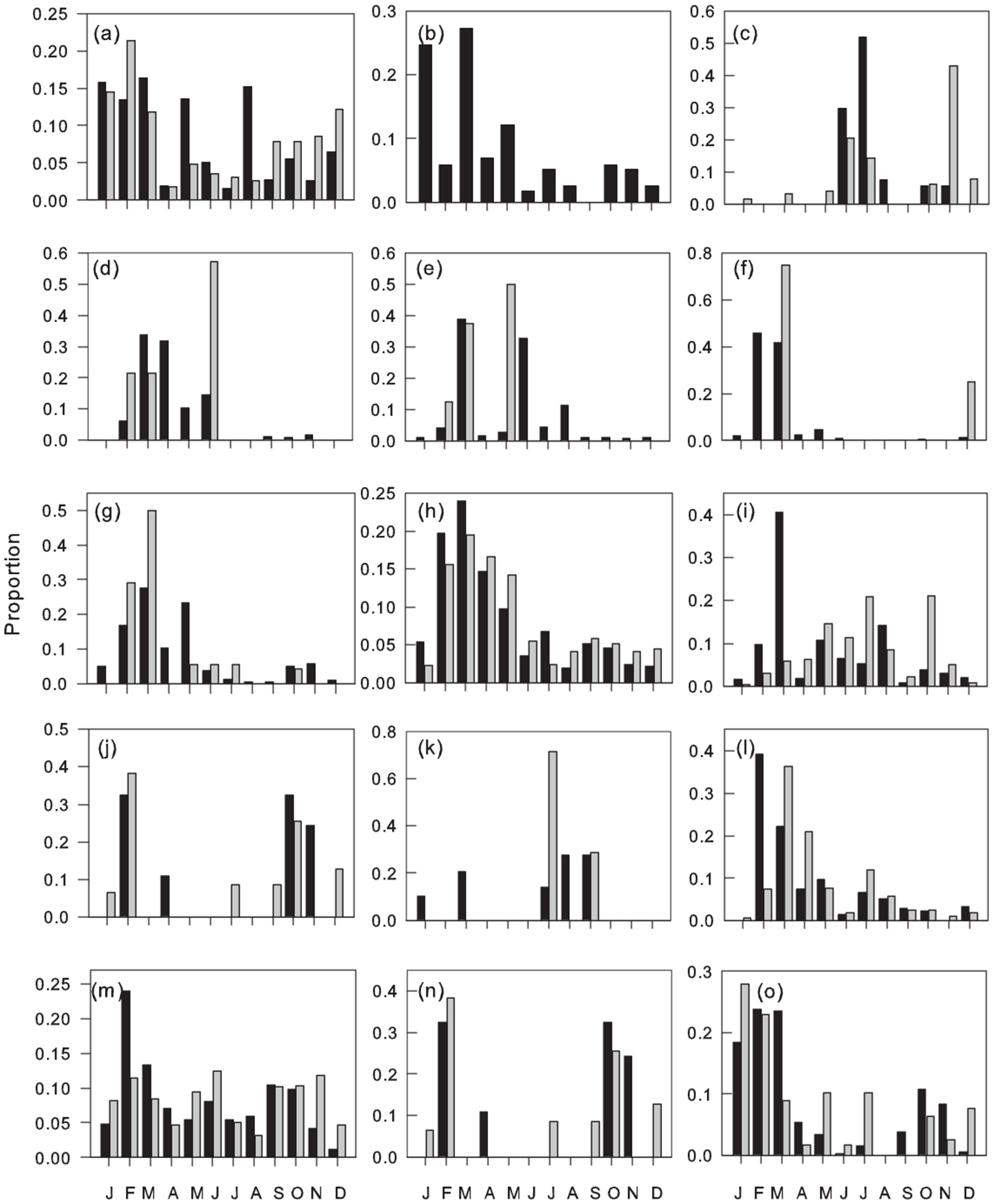
Figure 6. Monthly proportions of flights of Myrmicinae: (a) Tetramorium sericeiventre, (b) Tetramorium sp6 and of Formicinae, (c) Camponotus aberrans, (d) C. brutus, (e) C. foraminosus, (f) C. maculatus, (g) C. olivieri, (h) C. vividus, (i) Polyrhachis decemdentata, (j) P. laboriosa, (k) P. militaris, (l) Oecophylla longinoda, (m) Paraparatrechina albipes, (n) Plagiolepis cf. alluaudi, (o) P. brunni. Males (black columns), gynes (grey columns).
Seventeen species/morphospecies were caught significantly (P < 0.05) more frequently on rainy than on dry dates (table 2), and a further nine followed a similar trend with P < 0.10 and > 0.05. Tetraponera aethiops was trapped 2.4× as frequently on wet than on dry dates but, although gyne and male data analysed separately did not achieve statistical significance (table 2), when both sexes were combined the association with rainy dates was highly significant (χ2 = 8.72, P = 0.003). Further data are needed in order to confirm flight behaviour for other non-significant correlations. No Dolichoderinae were found in the wet-fly group and Para. albipes alone among Formicinae. Similarly, 17 species/morphospecies were caught significantly more frequently on dry than on wet dates, with a further three Tapinoma spp. males showing a similar trend (table 3). Camponotus, Tapinoma, and Technomyrmex were the predominant genera in the dry-fly group.
Table 2. Correlations between ant flights on wet and dry dates; predominantly wet-fly taxa

† Total wet fly-dates/total wet dates: total dry fly-dates/total dry dates, from date of first capture.
Table 3. Correlations between ant flights on wet and dry dates; predominantly dry-fly taxa
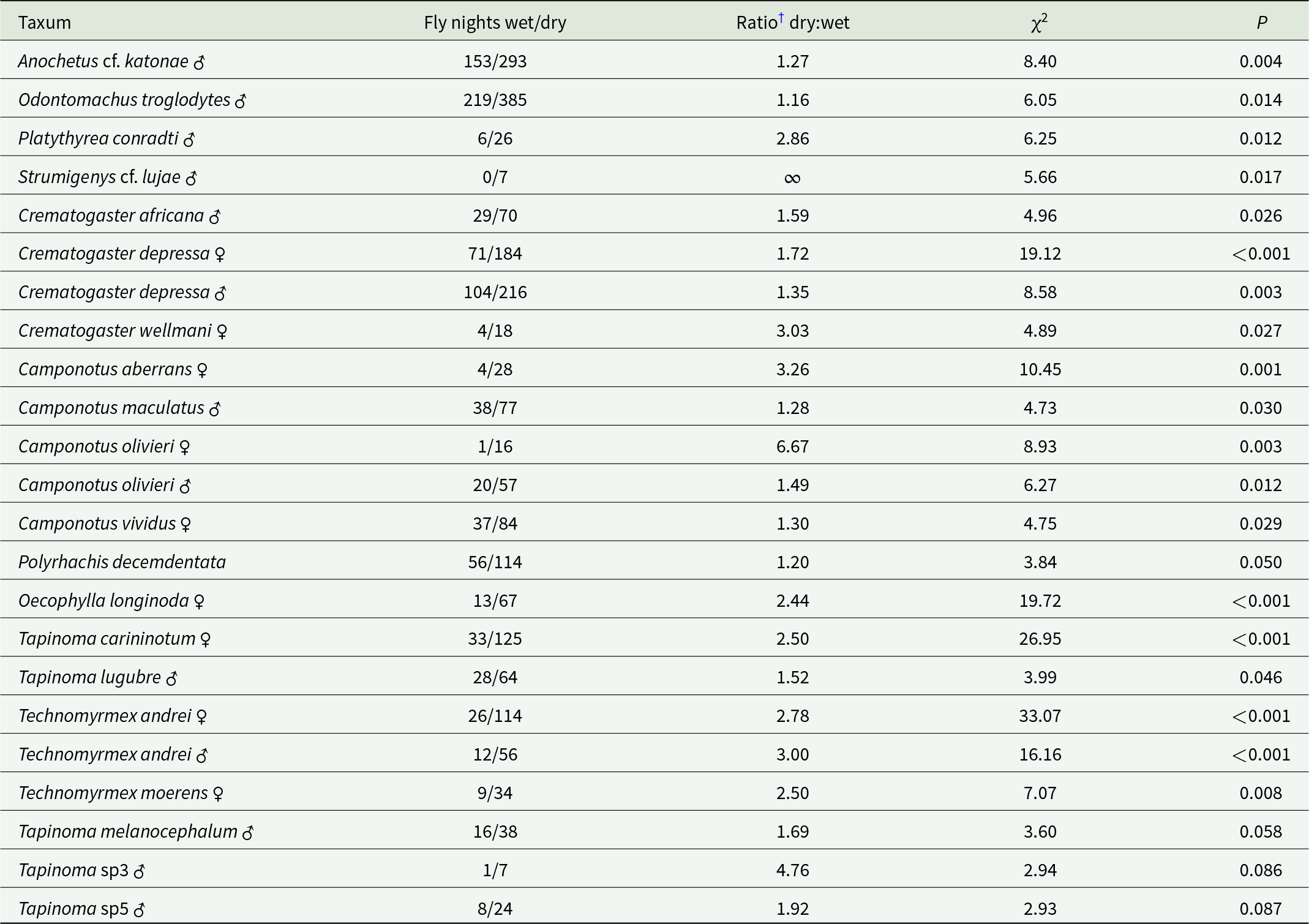
† Total dry fly-dates/total dry dates: total wet fly-dates/total wet dates, from date of first capture.
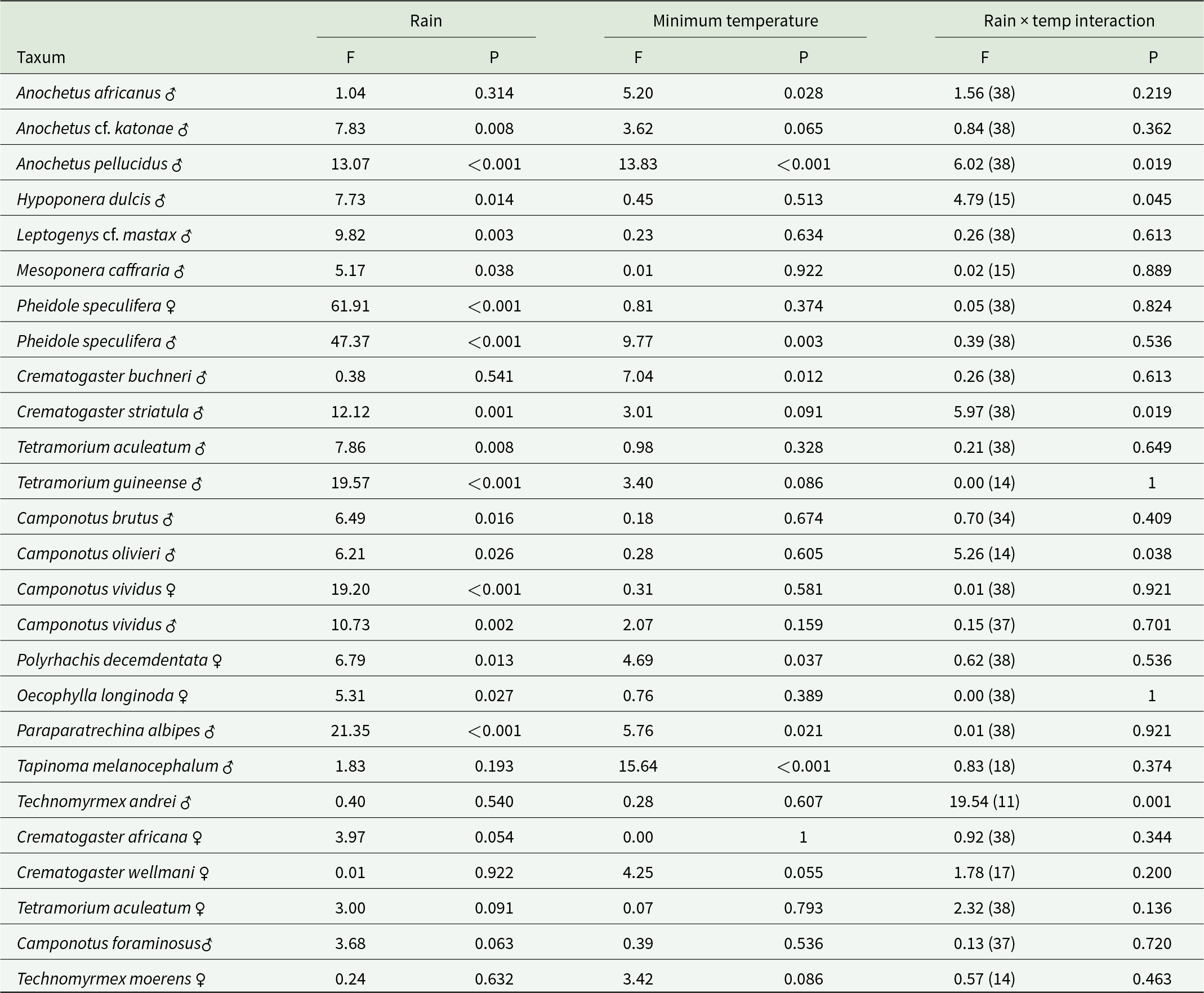
Either one or both sexes of fifteen species responded significantly positively to monthly rainfall, seven to temperature, five to the interaction between these two factors and a further five species responded to one or other of these factors with P < 0.10 but P > 0.05 (table 4). Further results and statistical tests on the effects of rainfall and temperature can be found in Supplementary material file S4.
Table 4. F-ratios and significances of regressions of monthly catches of winged ants with rain and minimum temperatures (error d.f. in parentheses)
Discussion
Robertson (Reference Robertson2000) speculated that 45–58% of African ant species are undescribed, while Fisher and Bolton (Reference Fisher and Bolton2016) suggest that current unknowns will probably average around 65%. Of the one hundred and thirty five species/morphospecies trapped in this study, 54 (40%) were unidentified beyond genus, so it is likely that some of the 28 species/morphospecies captured solely as males may be known and perhaps common, but are not yet associated with gynes or workers, a problem also encountered for much more thoroughly researched Neotropical species (Kaspari et al., Reference Kaspari, Pickering and Windsor2001b). For example, Ponerinae (genus indet.) sp1 was the ninth most numerous ant species/morphospecies collected, and second most numerous among Ponerinae, but as yet males have seemingly neither been described nor collected in association with workers. Cantone’s (Reference Cantone2017) keys could have facilitated providing generic names for these species, but the unnamed representative specimens lodged in the CRIG’s museum collection in 1978 by C.A.M.C. were absent by 2010.
Our study provides further evidence that, with the exception of some phasic Neotropical Dorylinae (Kannowski, Reference Kannowski1969; Nascimento et al., Reference Nascimento, Delabie and Della Lucia2011, Reference Nascimento, Delabie, Ferreira and Della Lucia2004; Rettenmeyer, Reference Rettenmeyer1963; Schneirla, Reference Schneirla1948, Reference Schneirla1972), the majority of common tropical ants across the five sub-families produce sexuals throughout the year, but with species-specific seasonal fluctuations in numbers, confirming conclusions for the few species monitored in Afrotropical light-trap studies (Bowden and Church, Reference Bowden and Church1973; Gibbs and Leston, Reference Gibbs and Leston1970; Leston, Reference Leston1979), nest inspections (Marchart, Reference Marchart1972; Nene et al., Reference Nene, Rwegasira, Nielsen, Mwatawala and Offenberg2016; Raignier, Reference Raignier1972; Raignier and van Boven, Reference Raignier and van Boven1955; Rwegasira et al., Reference Rwegasira, Mwatawala, Rwegasira and Offenberg2015; Way, Reference Way1954; Wheeler, Reference Wheeler1922) and more comprehensive studies from other regions (Fiala et al., Reference Fiala, Bin Hashim, Dumpert and Maschwitz2017; Kannowski, Reference Kannowski1991; Kaspari et al., Reference Kaspari, Pickering, Longino and Windsor2001a; Torres et al., Reference Torres, Snelling and Canals2001). However, nest studies do not necessarily equate with flight activity as sexuals may remain in the nest until specific environmental conditions, or physiological readiness (Peeters et al., Reference Peeters, Braun and Hölldobler2013; Peeters and Crewe, Reference Peeters and Crewe1986), trigger their emergence and dispersal (Hakala et al., Reference Hakala, Seppä and Helanterä2019; Peeters et al., Reference Peeters, Braun and Hölldobler2013; Staab and Kleineidam, Reference Staab and Kleineidam2014).
The flight periodicity of tropical Pseudomyrmecinae is unreported previously, although Deyrup and Trager (Reference Deyrup and Trager1986) in sub-tropical Florida reported flights of three Pseudomyrmex spp. from March to December. Ward (Reference Ward2022) in his monograph of Afrotropical Tetraponera lists the range of T. aethiops as from Nigeria to western Uganda, so we extend its known range further west.
Over 99.8% of Ponerinae captured here were males, which contrasts strongly with 27% males from a similar light-trap study in BCI (Kaspari et al., Reference Kaspari, Pickering and Windsor2001b). The sparsity of Ponerinae gynes trapped suggests that the reproductive phenology for the majority is principally female-calling (Hakala et al., Reference Hakala, Seppä and Helanterä2019; Helms, Reference Helms2018; Kaspari et al., Reference Kaspari, Pickering and Windsor2001b; Shik et al., Reference Shik, Donoso and Kaspari2013), as it is among Dorylus spp. (Hölldobler and Wilson, Reference Hölldobler and Wilson1990), and which is associated with male biased dispersal in which gynes, if winged, may fly short distances only or not at all (Hakala et al., Reference Hakala, Seppä and Helanterä2019; Helms, Reference Helms2018). Despite their absence from light-trap catches, museum records (Anon, 2024) confirm alate gynes for all four named Anochetus spp., Odontomachus assiniensis and Phrynoponera gabonensis, while Platythyrea conradti gynes are ergatoid (Molet and Peeters, Reference Molet and Peeters2006), as are those of Leptogenys mastax and probably L. zapyzis also, as most Leptogenys spp. have ergatoid queens (Schmidt and Shattuck, Reference Schmidt and Shattuck2014). Lenoir and Dejean’s (Reference Lenoir and Dejean1994) speculation that nuptial flights of Polyrhachis laboriosa and P. militaris are nocturnal is confirmed.
Rainfall and temperature are considered the main meteorological factors influencing ant nuptial flights, with rainfall considered the more important factor in tropical regions (Brown, Reference Brown, Meggers, Ayensu and Duckworth1973; Donoso et al., Reference Donoso, Basset, Shik, Forrister, Uquillas, Salazar-Mendez, Arizala, Polanco, Beckett, Diego Dominguez and Barrios2022; Kaspari et al., Reference Kaspari, Pickering, Longino and Windsor2001a; Torres et al., Reference Torres, Snelling and Canals2001; Uquillas et al., Reference Uquillas, Bonilla, Arizala, Basset, Barrios and Donoso2025). Both factors significantly affected flights here, with rainfall affecting 38 species/morphospecies, 28 positively and ten negatively, although Anochetus cf. katonae, Crematogaster africana males, P. decemdentata, Oe. Longinoda, and Plagiolepis brunni among the species/morphospecies affected positively flew significantly more frequently on dry dates in wet months. Peak flights for the majority of species/morphospecies were contemporaneous with the two rainy seasons, confirming earlier light-trap studies of female Oe. longinoda and Od. troglodytes (Gibbs and Leston, Reference Gibbs and Leston1970), and field observations of Oe. longinoda alone among African species (Booker, Reference Booker1961; Marchart, Reference Marchart1972; Nene et al., Reference Nene, Rwegasira, Nielsen, Mwatawala and Offenberg2016; Rwegasira et al., Reference Rwegasira, Mwatawala, Rwegasira and Offenberg2015; Way, Reference Way1954), and several species in the Americas (Kaspari et al., Reference Kaspari, Pickering, Longino and Windsor2001a; Pfeiffer and Linsenmair, Reference Pfeiffer and Linsenmair1997).
Low night temperatures inhibited flight in Nearctic (Baldridge et al., Reference Baldridge, Rettenmeyer and Watkins1980) and some Neotropical Dorylinae (Nascimento et al., Reference Nascimento, Delabie and Della Lucia2011; Tozetto et al., Reference Tozetto, Forrister, Duval, Hays, Garwood, Vargas Castro, Lattke, Sendoya and Longino2023), while rainfall was uncorrelated with flights. Similarly, temperatures below 25.9°C were found to inhibit flight in Atta vollenweideri (Myrmicinae), as does precipitation on a swarming day, although rainfall is essential on preceding days in order to stimulate mating flights (Staab and Kleineidam, Reference Staab and Kleineidam2014). On BCI, Uquillas et al. (Reference Uquillas, Bonilla, Arizala, Basset, Barrios and Donoso2025) found that more male ants (all species/morphospecies collectively) flew in the 2-week middle of the dry than in the two-week middle of the wet season, although Donoso et al. (Reference Donoso, Basset, Shik, Forrister, Uquillas, Salazar-Mendez, Arizala, Polanco, Beckett, Diego Dominguez and Barrios2022), using the same trap locations for a whole year, reported the highest catches of males coincided with the transition from the 5-month dry to the 7-month wet season. Males responded positively to increasing minimum temperatures in both BCI studies, and negatively to maximum monthly temperatures in Uquillas et al.’s (Reference Uquillas, Bonilla, Arizala, Basset, Barrios and Donoso2025) 16 year study, but positively to increasing daily maximum temperatures in Donoso et al. (Reference Donoso, Basset, Shik, Forrister, Uquillas, Salazar-Mendez, Arizala, Polanco, Beckett, Diego Dominguez and Barrios2022). Here low monthly night temperatures depressed catches for species/morphospecies of three Ponerinae, three Myrmicinae, two Formicinae and two Dolichoderinae, although neither monthly temperatures nor monthly and daily rainfall triggered flight activity of any Dorylinae, notwithstanding that collectively their peak flights coincided with peak rainfall (Fig. 2). Low monthly temperatures in wet months depressed catches of A. pellucidus, A. africanus, and H. dulcis.
Chamney (Reference Chamney1930) found that about 77% of the annual rainfall at Kumasi, 171 km WNW of Tafo, and sharing the same equatorial climate (Walker, Reference Walker and Wills1962), falls between 16.00 and 04.00 hours, hence mostly at night and contemporaneous with the light-trap’s operation. Although almost a century old, Chamney’s rainfall data probably remain applicable as Agyirifo and Otwe (Reference Agyirifo, Otwe and Oloyede2012) found that monthly rainfall distribution and volume at Kumasi was stable between the 30-year periods 1931–1960 and 1961–1990 while mean maximum monthly temperatures between those periods increased, but by less than 1 ºC which they attributed to local depletion of forest reserves.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0007485325000318.
Acknowledgements
We thank E. Ampomah for servicing the trap and for separating the ants from nightly catches of insects and Barry Bolton (NHMUK) for identifying some of the ant species.
Author contributions
M.B. initiated and executed the project until the end of May 1976, and C.A.M.C. thereafter. C.A.M.C. compiled the data, conducted the statistical analyses, and wrote the manuscript. Both authors approved the manuscript.
Competing interests
None.













