Introduction
Obsessive-compulsive disorder (OCD) is a chronic illness that, if left untreated, can significantly impact a person’s lifestyle, with a lifetime prevalence of approximately 1.6% globally and 0.76% in the Indian population.Reference Murthy 1 OCD can be treated through medication, psychotherapy, neurosurgery, and neuromodulation; however, a significant percentage of OCD sufferers do not improve during treatment.Reference Janardhan Reddy, Sundar and Narayanaswamy 2 First-line selective serotonin reuptake inhibitors (SSRIs) for OCD also increase the risk of adverse reactions because they need to be taken at high doses for several weeks before showing a clinically significant improvement. The availability of therapists, however, frequently limits cognitive behavior therapy; in low- to middle-income nations, there are shortages, whereas in high-income countries, there are lengthy waiting lists.Reference Stein and Lochner 3 , Reference Hollander, Allen and Steiner 4
Neuromodulation strategies, which involve influencing brain activity through noninvasive methods, have introduced new treatment possibilities for OCD. They work by modulating brain activity and are considered safe.Reference Janardhan Reddy, Sundar and Narayanaswamy 2 The technology used in transcranial direct current stimulation (tDCS) delivers a weak electrical current directly to the brain through 2 electrodes. Targeted cortical regions in the human brain may become more excitable or less excitable as a result of recurrent tDCS stimulation. Such neuromodulation approaches can impact different brain regions implicated in OCD. Target areas for neuromodulation in OCD patients who have the potential for therapeutic benefits have been identified at different regions, such as the orbitofrontal cortex, left dorsolateral prefrontal cortex (Lt. DLPFC), pre-supplementary motor area (pre-SMA), supplementary motor area (SMA), and right DLPFC.Reference Lefaucheur, Aleman and Baeken 5 , Reference Lefaucheur, Antal and Ayache 6 Regarding side effects, most associated with tDCS use are mild and transient. They often resolve on their own without specific interventions being required.Reference Rapinesi, Kotzalidis and Ferracuti 7 , Reference Bation, Mondino and Camus 8
Neuromodulation has been used as an early augmentation strategy along with ongoing serotonergic treatment in OCD previously.Reference Agrawal, Agarwal and Kar 9 , Reference Joshi, Kar and Dalal 10 However, the study by Joshi et al.Reference Joshi, Kar and Dalal 10 focused on the use of repetitive transcranial magnetic stimulation (rTMS) in adults with OCD, whereas the study by Agarwal et al.Reference Agrawal, Agarwal and Kar 9 focused on the use of early augmentation with tDCS in adolescents with OCD. When tDCS is used as an early augmentation in OCD treatment, patients may respond more quickly and recover sooner, leading to reduced impairment and enhanced functional improvement. This randomized controlled trial’s objective was to evaluate tDCS’s safety and effectiveness as an add-on treatment for drug-free adults with OCD. We postulated that, in comparison to fluoxetine alone, using tDCS as an adjunctive therapy would cause an early reduction in obsessive-compulsive symptoms.
Materials and Methods
Study design
This study was carried out in a tertiary care center in India using a randomized single-blind sham-control methodology. This study comprised adult patients (18-50 years old) who had been drug-free for the past 1 month, were diagnosed with OCD based on International Classification of Diseases - 10th Edition, Diagnostic criteria for research (ICD-10-DCR) criteria, and had a Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) score greater than 16. 11 Individuals with psychiatric disorders other than major depressive disorder, anxiety disorder, or tobacco use disorder, or those contraindicated for tDCS, were excluded. Patients with medical co-morbidities requiring priority management were also not included. Patients meeting the selection criteria were recruited into this study after providing written informed consent. At baseline, clinical variables were measured using several established scales: the Y-BOCS to rate the severity of OCD, the Dimensional Y-BOCS to assess the severity of OCD symptoms across different domains, the Hamilton Depression Rating Scale (HAM-D) for evaluating co-morbid depression, and the Hamilton Anxiety Rating Scale (HAM-A) to measure co-morbid anxiety. All patients were prescribed fluoxetine (20 mg per day) for 7 days to balance the effects of the medication. During the follow-up period, the dose of fluoxetine was increased from 40 to 60 mg per day. Rescue drugs such as tablet Clonazepam 0.25 mg (up to 1 mg per day) for anxiety and tablet Zolpidem (up to 10 mg per day) as per a need basis for insomnia were used. Ethical approval was taken from the Institutional Ethics Committee vide Letter No. 1913/Ethics/2023 with reference code XIV-PGTSC-IIA/P26 on January 19, 2023. This study was prospectively registered with the Clinical Trials Registry of India on February 13, 2023 (CTRI/2023/02/049682). Following registration, recruitment was done between February 2023 and February 2024, with follow-ups completed by March 2024.
Sample size
The sample size was calculated using the G*Power 3.1.9.7 application for statistical power analyses.Reference Faul, Erdfelder and Lang 12
The following parameters are taken into account for estimating the sample size.
F-tests: ANOVA—repeated measures, within-between interaction;
Analysis: a priori—compute required sample size;
Input: Effect size (f) = 0.25;
α err prob = 0.05;
Power (1 − β err prob) = 0.90;
Number of groups = 2;
Number of measurements = 3;
Corr among rep measures = 0.5;
Nonsphericity correction (ε) = 1;
Output: Noncentrality parameter (λ) = 13.5000000;
Critical F = 3.1316720;
Numerator df = 2.0000000;
Denominator df = 68.0000000.
The sample size that was determined was 36, and after considering a dropout rate of 10%, a final sample of 40 was estimated.
Randomization and blinding
A simple random sampling method was adopted. Participants meeting the inclusion criteria were assigned into active and sham tDCS groups in the allocation ratio of 1:1 by the computer-generated random table method. Participants were blinded to the allocation, whereas the investigator was aware of it. tDCS sessions were applied by the primary investigator himself. To mask the type of stimulation, patients in the sham group were also connected to tDCS electrodes, with a brief current applied for 10 seconds at both the beginning and end of the sessions to mimic the sensations experienced by the active treatment group. For the intervening 20 minutes, the electrodes remained connected, but no current was passed to the patients of the sham group.
tDCS protocol
The tDCS device Neurostim, made by Neurosoft in Russia, was used for stimulation. 13 To supply current, 2 electrodes measuring (5 cm × 5 cm) were positioned within a sponge measuring (7 cm×5 cm) and secured with rubber bands. To increase conductivity and lessen adverse effects, sponges were soaked in 0.9% saline. The cathode and the anode were placed on the left SMA and on the Lt. DLPFC, respectively. For stimulation, a 2-mA current was chosen. The active group underwent a 20-minute tDCS session. An extra 10 seconds were allocated for each ramp up and ramp down. In the sham group, the electrodes remained in the target areas for the full 20 minutes, but the current was passed only during the ramp-up and ramp-down phases. This process was carried out once a day until 10 sessions were finished. Appropriate precautions are taken to avoid transcranial shunting of electric current by ensuring separation between the electrodes and spillage of normal saline in the intervening scalp area between the 2 electrodes. The allotted time was 2 weeks. If individuals could not finish 10 sessions within 2 weeks, they were removed from this study. Patients in both groups were first prescribed 20 mg of fluoxetine daily, which was increased to 40 mg after a week. During the follow-up period, the fluoxetine dosage was further modified to 60 mg per day.
Clinical assessments of study variables
To rule out other psychiatric co-morbidity, MINI 6.0.0 was used to screen all the patients. 14 At baseline, the sociodemographic and clinical details of the patients were recorded using a semistructured proforma. Clinical symptoms, along with Y-BOCS, DY-BOCS, HAM-D, and HAM-A scores, were measured at baseline, 2 weeks, 4 weeks, and 6 weeks.Reference Hamilton 15 –Reference Fleck, Poirier-Littre and Guelfi 18 Using a tDCS side effect checklist, adverse events were measured and evaluated at each session.Reference Eryılmaz, Sayar and Ünsalver 19 Patients were treated conservatively if they had any mild side effects from tDCS.
Statistical data analysis
To analyze the data, IBM SPSS Software (version 24.0) was used. 20 Demographic and clinical variables were reported using descriptive statistics. The data were analyzed for normality using the Shapiro-Wilk test and were found to have a normal distribution. However, due to the small sample size, we applied nonparametric tests (Mann-Whitney U test). Fisher’s Exact test was used to compare categorical data. The baseline and post-intervention characteristics were compared between groups. To assess changes in outcome measures during the course of this study, repeated measures analysis of variance (ANOVA) was used. The effect size was calculated using Cohen’s d. P-values <0.05 were taken as statistically significant.
Results
The trial was carried out in 2023 following registration at the clinical trial registry of India. Out of 154 patients screened, 43 were enrolled in this study, and 111 were excluded. The most common reason for exclusion was the inability to attend daily tDCS sessions (n = 41). Of the 43 participants enrolled, 3 did not complete the full intervention of 10 tDCS sessions (dropped out after the very first session and withdrew consents)—1 from the active tDCS group and 2 from the sham tDCS group. All 40 patients completed the intervention (20 in each group). In the active group, 3 patients were lost to follow-up—2 at the 4-week assessment and 1 more at the 6-week assessment. In the sham group, 4 patients were lost to follow-up—3 at the 4-week assessment and 1 more at the 6-week assessment. For this study, we have performed an intention-to-treat analysis, so the analysis of patients lost to follow-up was done using the last observation carried forward method (Figure 1). Baseline demographic characteristics and clinical variables (Table 1) of both groups were comparable.
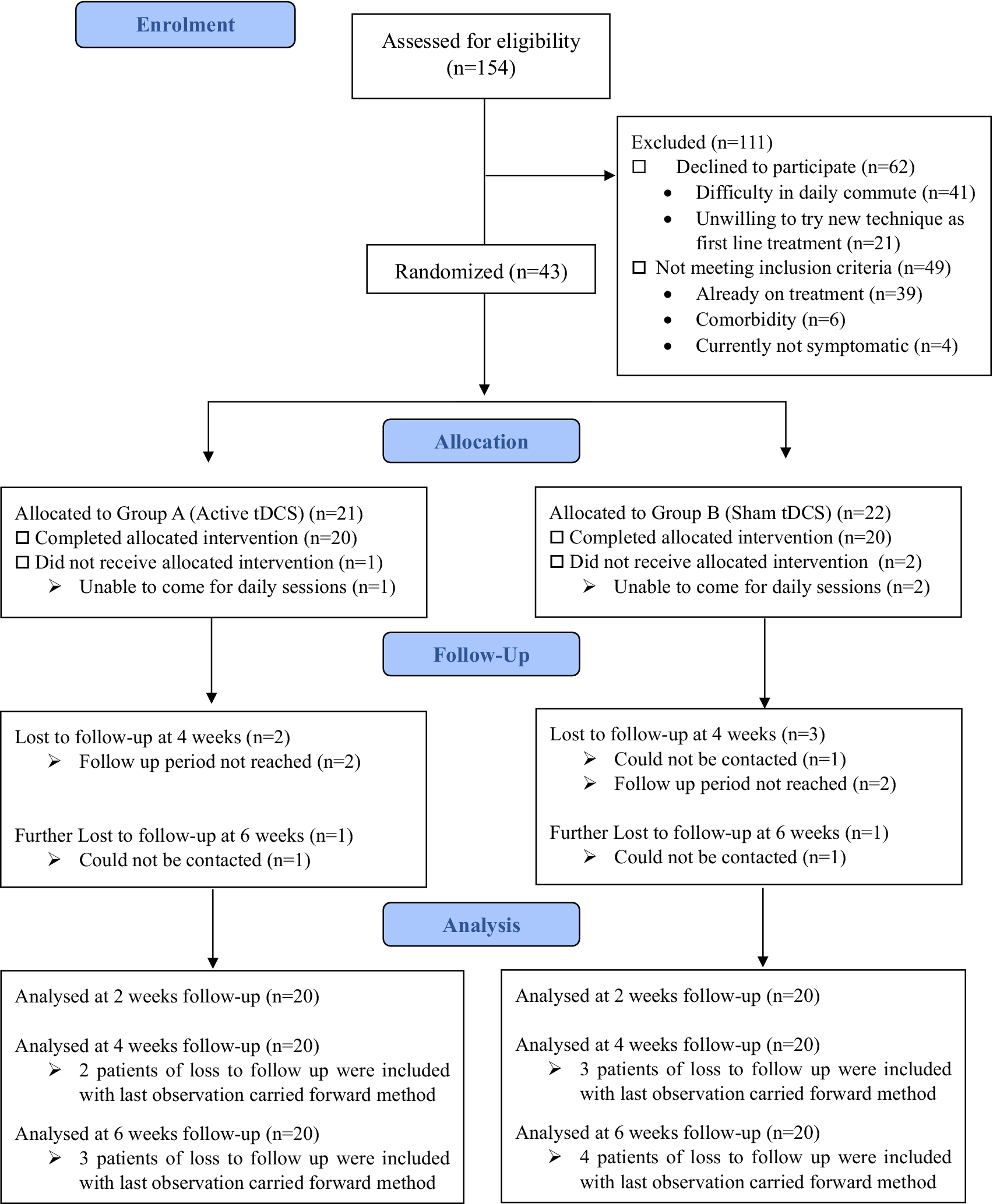
Figure 1. Consolidated standards of reporting trials (CONSORT) flow diagram for this study.
Table 1. Sociodemographic and Clinical Variables of the Patients
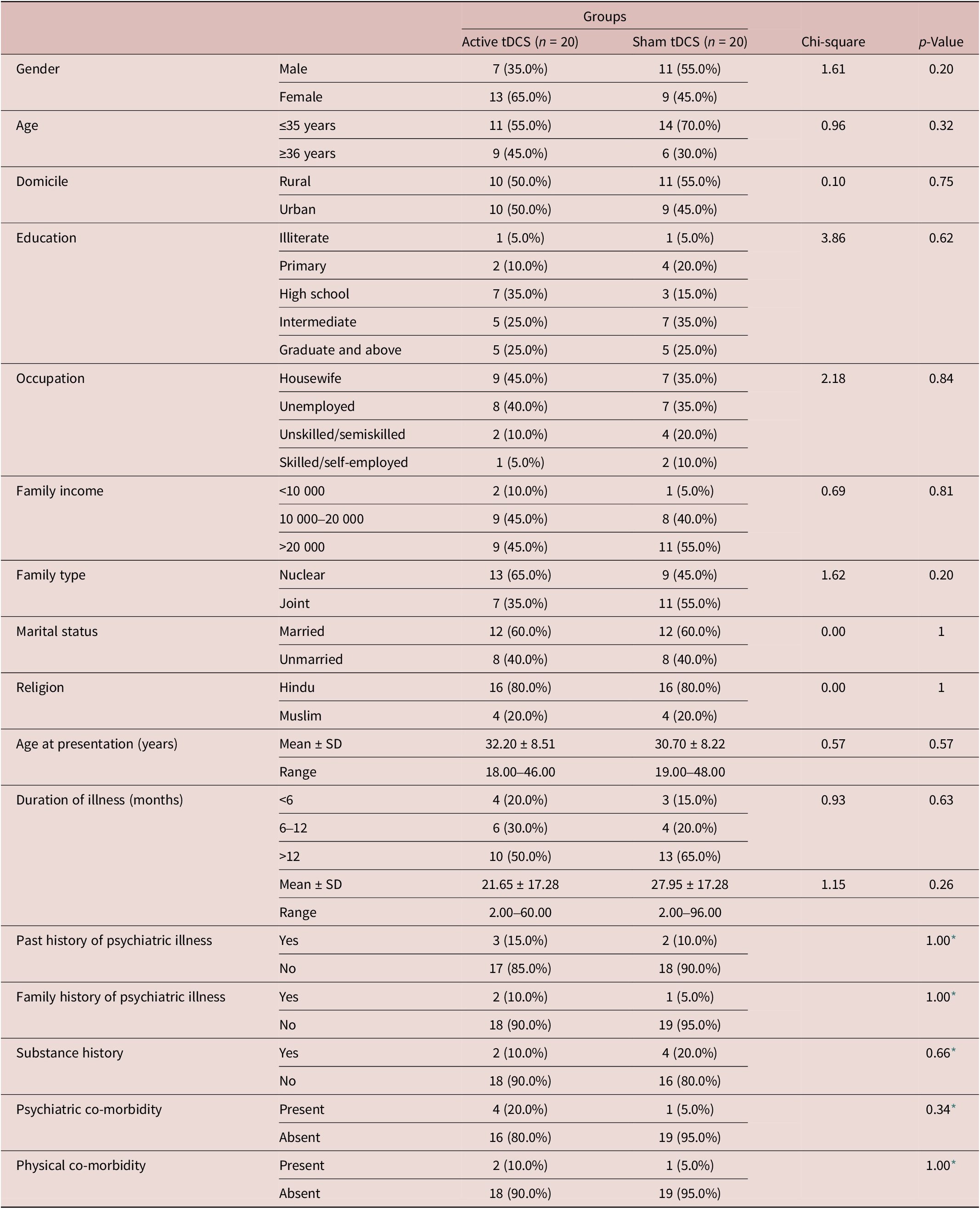
Abbreviation: tDCS, transcranial direct current stimulation.
* Fischer exact test and p-value.
In the active group, all 20 patients had obsessions related to contamination and cleaning, while 3 had obsessions in the symmetry domain and 2 in the sexual and religious domains. In the sham group, 18 patients had obsessions related to contamination and cleaning, 2 had symmetry obsessions, 1 had aggressive obsessions and related compulsions, and 2 had sexual and religious obsessions.
Outcome measures
The total Y-BOCS score at baseline was significantly higher in the active group (Mean ± SD = 26.10 ± 2.53) compared with the sham group (24.10 ± 2.57), with a U-value of 2.187 and a p-value of 0.029. The total Y-BOCS score at 2 weeks was lower in the active group (Mean ± SD = 21.70 ± 2.39) compared with the sham group (Mean ± SD = 23.10 ± 2.43); however, this difference was not statistically significant. At 4 and 6 weeks, the Y-BOCS scores (including obsessions, compulsions, and total scores) were lower in the active group compared with the sham group, with statistically significant differences. Post hoc analysis showed a change in the mean reduction of total Y-BOCS scores across different assessment points in both groups. There was a significant difference in the mean reduction of total Y-BOCS scores from baseline to subsequent follow-up between the groups (Table 2).
Table 2. Comparison of Y-BOCS Total Score at Baseline, 2 Weeks, 4 Weeks, and 6 Weeks in between the Groups
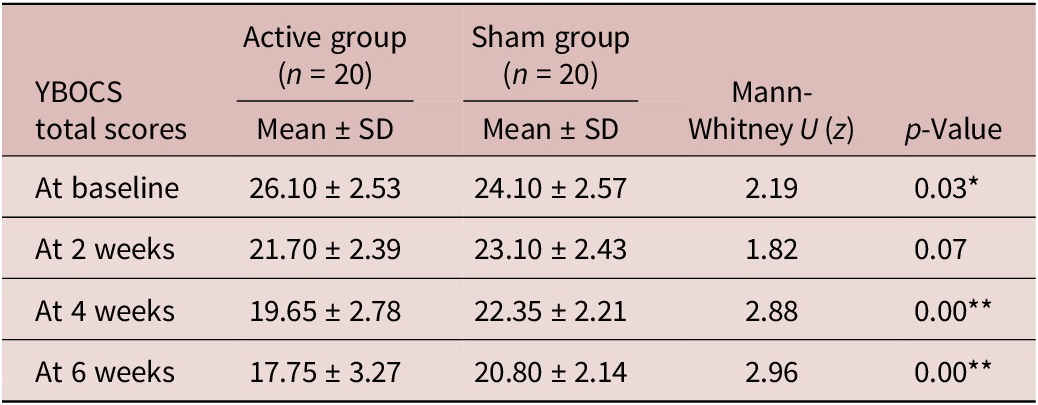
* refers to significance at the level of p value <0.05.
** refers to significance at the level of p value <0.01.
The active tDCS group exhibited higher HAM-D scores at baseline and follow-ups, but these differences were not significant. Post hoc analysis was done to show the change in the mean reduction of HAM-D scores between both the active and sham groups. There was a significant difference in the mean reduction of HAM-D scores between baseline and 4 weeks (p = 0.027) and between 2 weeks and 4 weeks (p = 0.013) (Table 3).
Table 3. Comparison of Mean Reduction in Y-BOCS Total Score and HAM-D between 2 Groups across Different Assessment Points
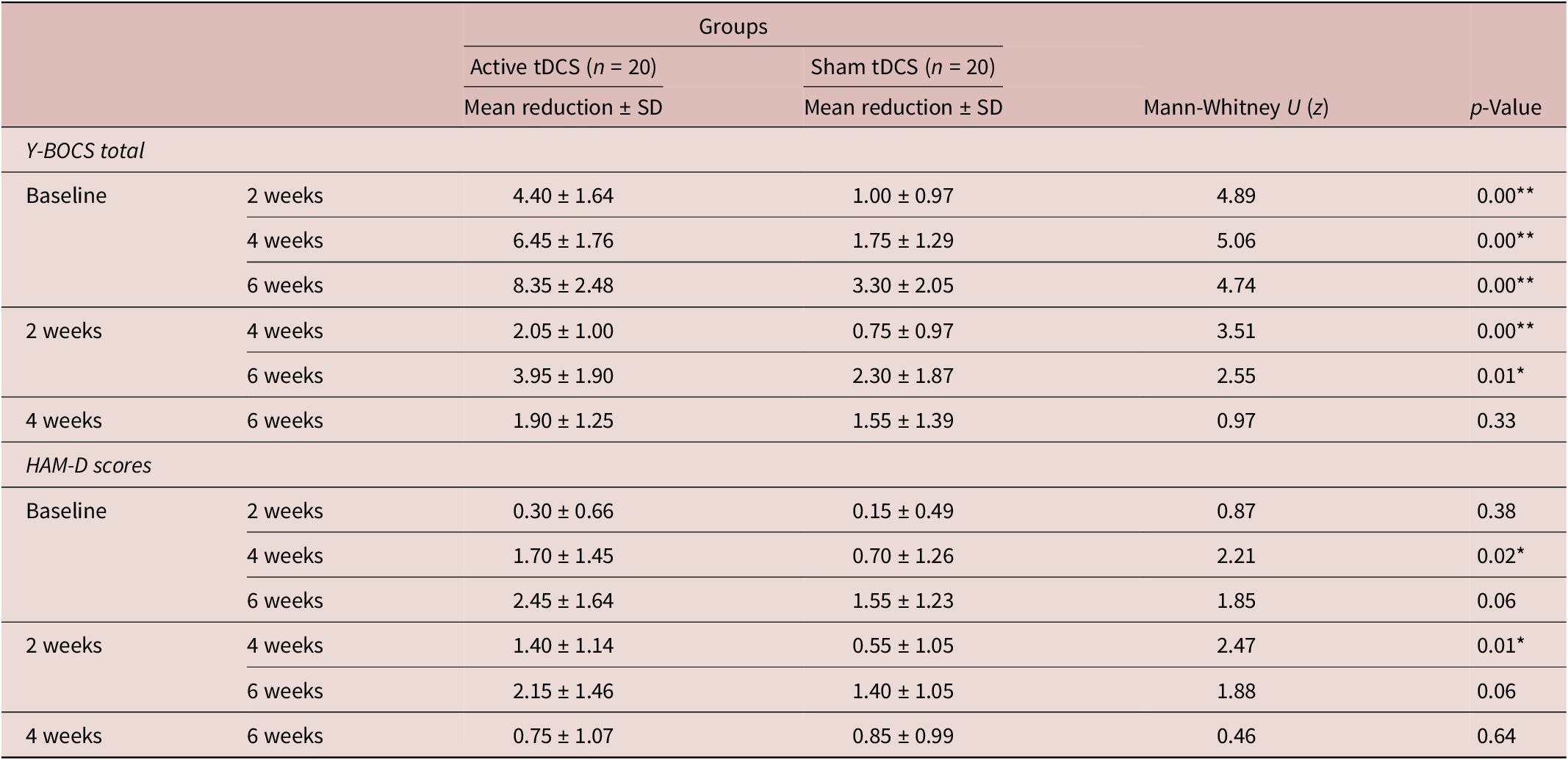
* refers to significance at the level of p value <0.05.
** refers to significance at the level of p value <0.01.
The active tDCS group exhibited higher HAM-A scores at baseline and follow-ups, but these differences were not significant. Post hoc analysis was conducted to assess the change in the mean reduction of HAM-A scores between the 2 groups. No significant differences in the mean reduction of HAM-A scores were observed across any time points, as all p-values were above 0.05.
The partial response rates at 2, 4, and 6 weeks are 10% (2/20), 40% (8/20), and 35% (7/20) for the active group, respectively, with the sham group showing a partial response rate of 5% (1/20) at 6 weeks and 0% at both 2 and 4 weeks. The response was noted in 5% (1/20) of patients at 4 weeks and 40% (8/20) at 6 weeks, exclusively in the active tDCS group. No responses were observed in the sham group at any time point.
The number needed to treat (NNT) for response at 6 weeks was 2.5 (95% confidence interval [CI]). The effect size of the intervention at 2 weeks, after the completion of 10 sessions of tDCS, was calculated to be 0.58, indicating a moderate effect size according to Cohen’s d.
Safety measures
In this study, patients in the active group reported a higher intensity of side effects compared with those in the sham group, although no major adverse effects were recorded. The most commonly encountered side effects included headache (5.5% in the active group vs. 5.0% in the sham group), numbness at the stimulation site (3% vs. 1.5%), and pain at the stimulation site (5.0% in both groups). The overall incidence of any side effects was 13.5% in the active group and 12.5% in the sham group. The relative risk of experiencing any side effect was 1.08 times more likely in patients receiving an active tDCS session.
The number needed to harm was calculated at 100, indicating that for every 100 sessions, there would be one additional adverse reaction. This suggests that tDCS is a safe neuromodulation technique.
Discussion
The current study was a 1-year, randomized, controlled, single-blind trial at a tertiary psychiatry center in North India, aiming to evaluate the safety and efficacy of tDCS as an early augmentation technique alongside standard anti-obsessional medication in adult patients with OCD. The findings provide strong evidence that tDCS, when combined with fluoxetine, may accelerate the reduction of obsessive-compulsive symptoms, indicating its potential as an effective adjunctive treatment for OCD.
Both the groups were compared on clinical variables at baseline, including past and family history of psychiatric illness, substance history, and psychiatric and physical co-morbidities. Most of the patients had a duration of illness of more than 1 year in both the groups. The mean duration of illness was higher in the sham group (27.95 years) compared with the active group (21.65 years). Individuals with an extended duration of illness at the outset typically exhibit a less favorable prognosis.Reference Reddy, Alur and Manjunath 21
In this study, in line with previous studies targeting the SMA and Lt. DLPFC, our protocol used cathodal stimulation over the SMA and anodal stimulation over the Lt. DLPFC. These regions have consistently been implicated in the pathophysiology of OCD and have shown a strong evidence base for targeting OC symptoms, and our results further validate their use as targets for neuromodulation.Reference Joshi, Kar and Dalal 10 , Reference Harika-Germaneau, Heit and Chatard 22 –Reference Zhong, Ou and Chen 24 However, another Indian study used anodal tDCS with 2 sessions per day for 5 consecutive days targeting pre-SMA and found a significant reduction in the symptoms of OCD.Reference Gowda, Narayanaswamy and Hazari 25
The active tDCS group demonstrated a significant reduction in depressive symptoms from baseline to 4 weeks, indicating its potential antidepressant effects. These findings suggest that tDCS, as an augmentation strategy, could be effectively used in treating OCD patients, particularly those with co-morbid depression, to help alleviate depressive symptoms.
In OCD, when compared to the initial assessment, a reduction of at least 35% in Y-BOCS scores is considered a response, a reduction of at least 25% but less than 35% is considered a partial response, and achieving a Y-BOCS score of 12 or lower is considered remission. Reference Mataix-Cols, de la Cruz and Nordsletten 26 Using this set of criteria, we found that 8 patients (40%) in the active group responded after 6 weeks, whereas no patients in the sham group responded at all during the course of this study. In an open-label trial, 15% of individuals showed an improvement of more than 35% in their Y-BOCS scores after just 1 week of receiving 20 sessions of cathodal stimulation of the SMA with tDCS.Reference Kumar, Kumar and Verma 23 The higher response rate observed in our study may be attributed to the exclusion of treatment-resistant patients.
All participants in this study tolerated the intervention well, with no significant adverse events or serious complications observed, and no participants withdrew from the trial due to side effects. Headache followed by pain at the stimulation site and numbness at the stimulation site were the predominant side effects. The side effects associated were transient and tolerable. Hence, tDCS can be considered a safer modality.
The intervention’s effect size was 0.58 at 2 weeks following the completion of 10 tDCS sessions, indicating a moderate effect and suggesting that the intervention had a meaningful impact on the target outcomes. A similar effect size of 0.54 was reported in a meta-analysis of 22 studies, reinforcing the consistency of the findings with previous research.Reference Zhou and Fang 27 Our study found a low NNT of 2.5 to achieve a response at 6 weeks (95% CI), suggesting that the intervention is effective. This is consistent with a similar NNT of 3 reported in a randomized, double-blind, sham-controlled trial involving resistant adult patients with OCD.Reference Gowda, Narayanaswamy and Hazari 25 The observed decrease in Y-BOCS scores over multiple time periods in the active group with transient side effects, together with the moderate effect size and low NNT, highlights the clinical potential of tDCS in expediting symptom treatment for OCD patients.
Strengths and limitations
Globally, there has been limited research on the use of neuromodulation as an early augmentation technique for treating OCD. To the best of our knowledge, this is one of the first studies in India to employ tDCS as an early augmentation technique in adult patients with OCD. Stringent selection criteria were followed for the recruitment of patients. Consistency among the groups was ensured by administering only one anti-obsessional medication (fluoxetine). Because this study’s power is 90%, the findings are more reliable.
This study’s small sample size and single blinding may limit the reliability and generalizability of its findings. Additionally, the short follow-up period of only 6 weeks prevents any assessment of the long-term effects of tDCS. The results may also be restricted in generalizability due to the majority of participants being from a single center. The majority of the current studies on tDCS in OCD are highly heterogeneous in their protocols as reported in a recent systematic review and Consolidated Standards of Reporting Trials (CONSORT) evaluation.Reference Green, Loftus and Anderson 28 There is a need for studies with more homogeneous protocols and larger sample sizes to better interpret the effectiveness of tDCS in OCD.
Conclusion
Early augmentation using tDCS is a safe and effective strategy for the early reduction of symptom severity in OCD. This could avoid the lag period of SSRI to achieve a response. Further research in a larger sample is required to substantiate the evidence.
Financial support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.





