Background
In ecology, the intermediate disturbance hypothesis (IDH) states that species diversity in ecosystems is maximized when the level of disturbance is neither too low nor too high (Vandermeer et al. Reference Vandermeer, Boucher, Perfecto and de la Cerda1996). The hypothesis is based on the following central premises: (1) ecological disturbances have measurable effects on species diversity within an ecosystem; (2) interspecific competition can cause colonizing species (r) to drive species with low growth and reproduction rates (k) to extinction; and (3) moderate disturbances prevent interspecific competition (Roxburgh et al. Reference Roxburgh, Shea and Wilson2004). Thus, the IDH postulates that, at low levels of disturbance, the most competitive species can drive subordinate species to extinction and can dominate the ecosystem. Conversely, at high levels of disturbance, all species are at risk of extinction. Finally, at intermediate levels of disturbance, species diversity can be maximized because species that thrive in both early and late successional stages coexist (Bendix et al. Reference Bendix, Wiley and Commons2017).
More than 1000 articles in the Web of Science refer to the IDH in one way or another (Moi et al. Reference Moi, García-Ríos, Hong, Daquila and Mormul2020). Researchers began using the IDH in the 1980s, and its use peaked between 2008 and 2015. The IDH has been used in many fields of ecology, from microbiology to environmental science, with landscape ecology being the field with its greatest use (Moi et al. Reference Moi, García-Ríos, Hong, Daquila and Mormul2020). Wilkinson (Reference Wilkinson1999) recognized that concern about the relationship between ecosystem disturbance and species richness dates back to the 1940s with the work of ecologists such as Arthur Tansley, Alex Watt and William Eggeling. It was not formalized until the 1980s, following studies by Grime (Reference Grime1973) on competition in herbaceous plants, Horn (Reference Horn1975) on forest succession and Connell (Reference Connell1978) on animal diversity and coral reefs (see Wilkinson Reference Wilkinson1999); Connell is credited with coining the hypothesis.
Currently, the IDH is an integral part of out-of-equilibrium ecological models that challenge classical competitive exclusion theories. The IDH is supported by studies of tropical rainforests, temperate grasslands and coral reefs, which exhibit higher diversity in areas affected by natural disturbances such as fire, hurricanes or storms when compared to undisturbed areas (Sheil & Burslem Reference Sheil and Burslem2013). The IDH has been criticized, however, for its application to small assemblages under limited disturbance regimes and small geographical areas, making it difficult to extrapolate to broader ecological scales (Fox Reference Fox2013). Similarly, studies such as Lehmann et al. (Reference Lehmann, Archibald, Hoffmann and Bond2011) challenge the linear approaches of the IDH, demonstrating the complications involved in attributing unique causalities to low, medium or high levels of disturbance on biodiversity, especially when other complex ecological and biophysical variables are also involved in the assemblage of species in an ecosystem.
We propose to reevaluate the challenges faced by the IDH in ecology by incorporating new knowledge regarding the intentionality, spatial scale, duration and non-linearity not of disturbances, but of the mediations carried out by Homo sapiens. This requires an approach that empirically documents complex human–environment relationships, highlighted by biocultural factors. Our objectives are: (1) to advance the ecological understanding of the intervention–biodiversity relationship through a biocultural lens; (2) to characterize and illustrate human–environment mediations (H-EMs) that maintain and even increase biodiversity levels; and (3) to consider the implications of developing collaborative research programmes based on a biocultural hypothesis. These objectives will address conservation policies and natural resource management schemes to promote the recognition and incorporation of the biocultural legacies of human societies.
Arguments: an alternative biocultural hypothesis
A major reason why the IDH is challenged is its lack of clarity in defining the intensity, duration and spatial scale of disturbances. We propose to broaden the strictly ecological framework by incorporating the contributions that ethnoecology, ethnobotany, anthropology, human geography, historical ecology, archaeology and agroecology have made to the understanding of the relationship between human management and biodiversity levels. These contributions to the understanding of ‘disturbances’ from the reciprocal and complex interrelationships of humans and the environment call for a paradigmatic shift derived from biocultural theory (Nietschmann Reference Nietschmann1992, Posey Reference Posey and VD1999, Maffi Reference Maffi2005, Toledo & Barrera-Bassols Reference Toledo and Barrera-Bassols2008). As part of this paradigmatic transition, the biocultural axiom posits that ecological and cultural richness are interdependent and geographically co-terrestrial, as evidenced by the overlap of biological, agricultural and linguistic diversity (Loh & Harmon Reference Loh and Harmon2005). The primary units of analysis are biocultural landscapes, defined as discrete spatial assemblages where ecological processes, production systems and natural resource management practices, including technologies, social organization forms and ontological systems, coalesce and accumulate over time (Pérez-Balladares & Farfán-Heredia Reference Pérez-Valladares and Farfán-Heredia2024). A significant outcome of these complex interactions that bear on the IDH is the resulting diversity of species and landscapes (Toledo et al. Reference Toledo, Barrera-Bassols and Boege2019).
Based on the biocultural axiom, we propose the concept of H-EMs. H-EMs are described as non-degrading, constructive interventions that humans historically created across their landscapes. Historical ecologists point out that these mediations result from the synergies of human subsistence interactions with their environment based on selective pressures affecting the evolution of both humans and other species over centuries to millennia (Balée Reference Balée2006), as well as in reaction to sudden disturbance dynamics such as the colonization of new environments (Bray et al. Reference Bray, Hahn, Lourdusamy and Saraiva2023). H-EMs are generally characterized by spatial and temporal adaptations in: (1) long-lasting histories (longue durée) driven by dynamic and flexible responses to environmental changes; (2) human niche modifications and constructions interacting with the environment; and (3) landesque and laboresque investments based on environmental knowledge (Odling-Smee et al. Reference Odling-Smee, Erwin, Palkovacs, Feldman and Laland2013, Håkansson & Widgren Reference Håkansson and Widgren2014, Boivin et al. Reference Boivin, Zeder, Fuller, Crowther, Larson and Erlandson2016). Landesque and laboresque investments include accumulated practices, technologies, techniques, institutions and worldviews that lead to productive, flexible, sustainable environments (Antorcha-Pedemonte et al. Reference Antorcha-Pedemonte, Rivera-Núñez and Fargher Navarro2023, Ford Reference Ford, McLeester and Casana2024).
We identified six major types of interlinked H-EMs operating at different environmental scales: (1) pyrodiversity ecology; (2) agroforestry and silvopastoral management; (3) human-made soils and mineral and biological enrichment; (4) species translocation, selection and domestication; (5) ecological co-evolutionary dynamics; and (6) microclimatic patterns (Table 1). These H-EM types are prevalent in regions of high biocultural diversity or anthropobiomes (Ellis & Ramankutty Reference Ellis and Ramankutty2008), such as West and Central Africa, the Indomalayan region, Oceania and the Pacific Islands, the Amazon Basin, the Andean zone and Mesoamerica (Loh & Harmon Reference Loh and Harmon2005). Our analysis focuses on examples from Mesoamerica, the region where our own research efforts have been concentrated (Fig. 1).
Table 1. Characterization of the main human–environment mediations (H-EMs), featuring but not limited to intertropical regions with central scales of operation indicated.
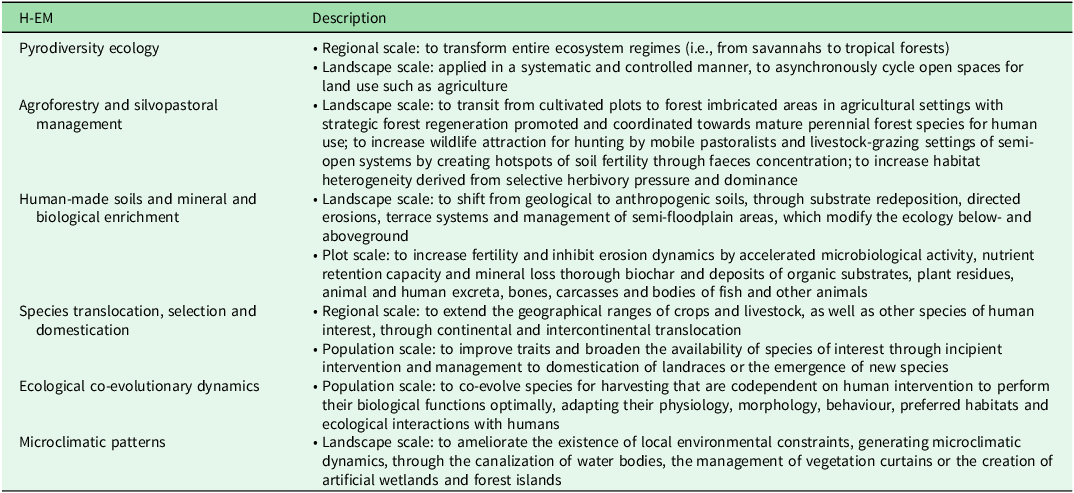

Figure 1. Examples of human–environment mediations (H-EMs) in Mesoamerican and Amazonian biocultural landscapes. (a) Yucatecan Maya peasant specializing in controlled, low-temperature fire for agricultural management (‘wind-tender’). (b) Terra preta do indio, a biologically and mineral-enriched anthroposol in the Brazilian Amazon. (c) Yucatecan Maya forest mosaic dominated by ramon trees (Brosimum alicastrum), known as the ‘old village forest’, vital for ancient Maya nutrition. (d) Ch’ol Maya peasant in Chiapas crafting a trap for wild turkey (Agriocharis ocellata) and white-tailed deer (Odocoileus virginianus), adapted to agricultural successional regeneration. (e) Ancient wetland agriculture system using artificial raised fields (chinampas) in the Basin of Mexico, supporting endemic species such as the Mexican axolotl (Ambystoma mexicanum). (f) Yucatecan Maya home garden with over 600 plant and animal species, selected from the tropical forest, surrounding the domestic unit.
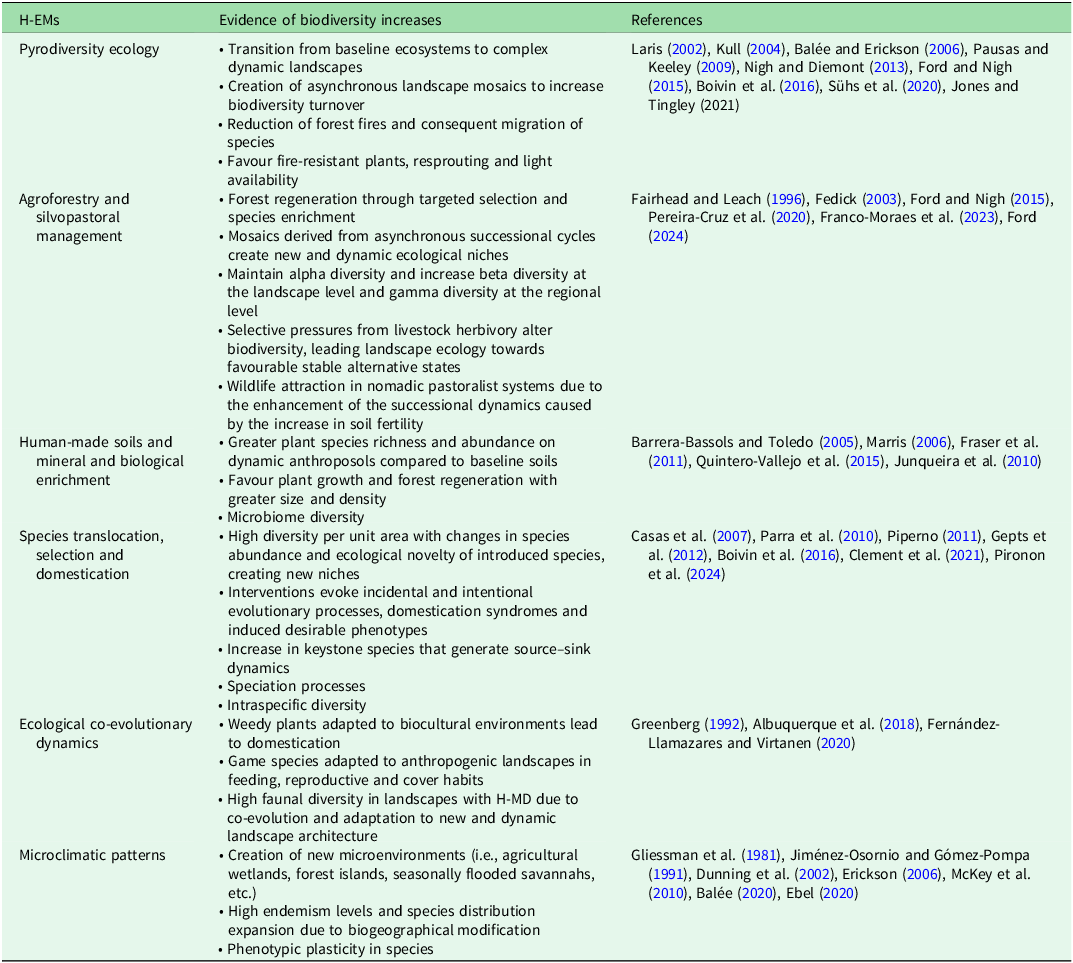
Numerous studies over the past few decades have shown that H-EMs can enhance biodiversity in biocultural landscapes, particularly – but not limited to – tropical forests (Boivin et al. Reference Boivin, Zeder, Fuller, Crowther, Larson and Erlandson2016, Rivera-Núñez et al. Reference Rivera-Núñez, Fargher and Nigh2020, Clement et al. Reference Clement, Casas, Parra-Rondinel, Levis, Peroni and Hanazaki2021, Levis et al. Reference Levis, Flores, Campos-Silva, Peroni, Staal and Padgurschi2024). Research conducted in the Amazon Basin, Mesoamerica and West and Central Africa (Table 2) focuses on these regions with the highest concentrations of biodiversity (Mittermeier et al. Reference Mittermeier, Myers and Goettsh Mittermeier2000). These areas, along with the Indomalayan region, the Andean zone and Oceania and the Pacific Islands, have the highest richness of useful plants per unit area and high levels of plant endemism (Pironon et al. Reference Pironon, Ondo, Diazgranados, Allkin, Baquero and Cámara-Leret2024). Overall, the cumulative research summarized in Table 2 shows that H-EMs can increase biodiversity levels in managed landscapes relative to reference ecosystems, especially with respect to beta and gamma biodiversity, often without significant changes in alpha diversity (Mayor et al. Reference Mayor, Cahill, He, Sólymos and Boutin2012, Albuquerque et al. Reference Albuquerque, Gonçalves, Júnior, Chaves, da Silva Oliveira and da Silva2018).
Table 2. Implications of human–environment mediations (H-EMs) in the generation of biodiversity peaks: findings in intertropical areas.
Based on H-EMs, as an alternative biocultural hypothesis to the ecological approach of IDH, we postulate that human mediations could produce highly biodiverse landscapes that are frequently of long temporal duration and occurring at broad cumulative geographical scales, and that work with complex natural processes to modify landscapes without degradation. Biocultural landscapes often exhibit greater biodiversity than reference ecosystems with minimal human activity and surpass ecosystems with natural or anthropogenic intermediate disturbances, where early and late successional species confluence has peaked (Fig. 2).
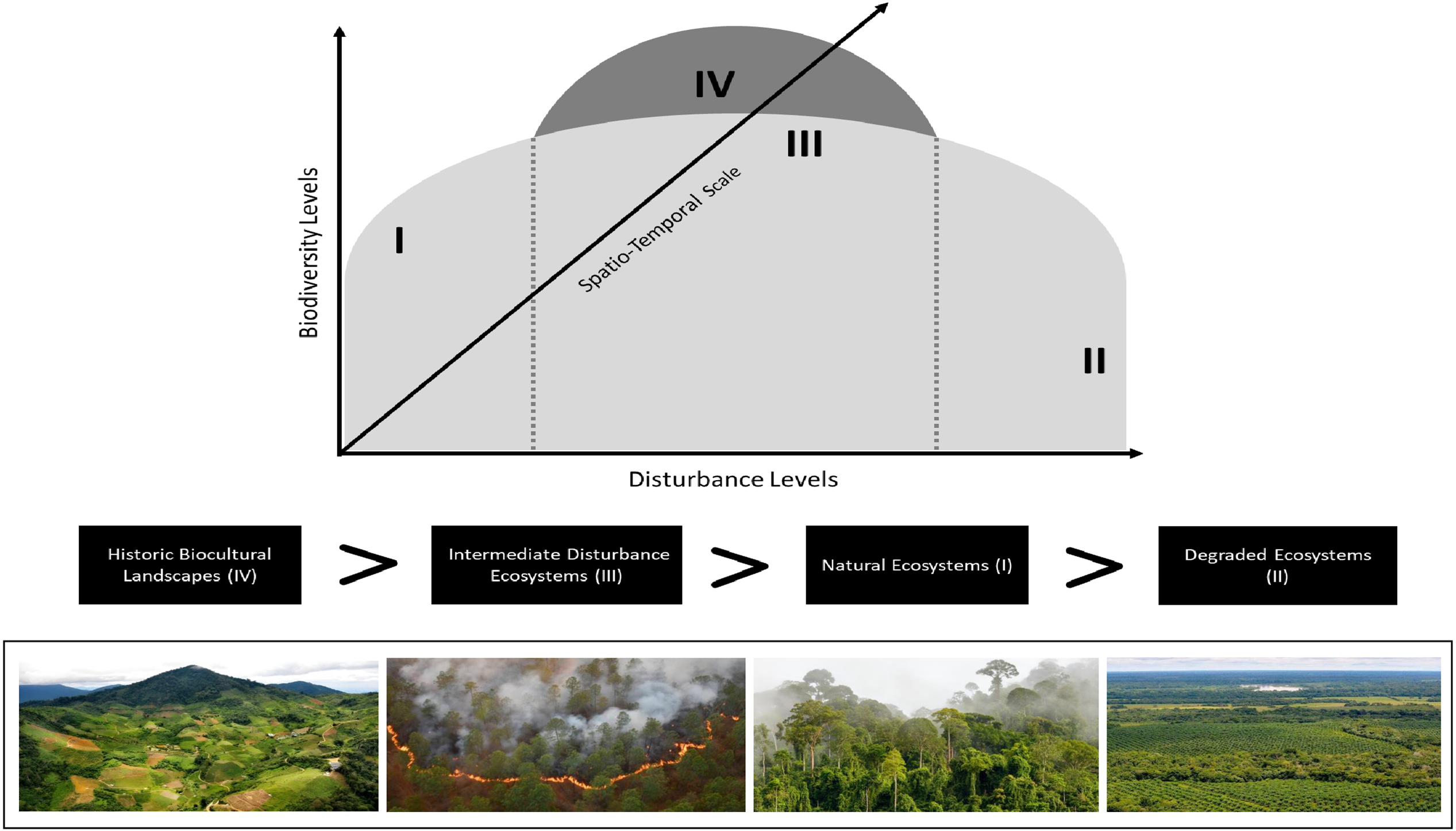
Figure 2. Schematization of the biocultural hypothesis of human–environment mediations (H-EMs) and biodiversity peaks represented by a ‘hump-backed’ model. Scenarios I–III correspond to the classic intermediate disturbance hypothesis (IDH) model. Scenario I: at low levels of ecological disturbance, species richness decreases due to competitive exclusion. Scenario II: at high levels of disturbance, species richness decreases due to increased species migration and/or extinction. Scenario III: at intermediate levels of disturbance, diversity increases because species adapted to both early and late successional stages can coexist. Alternative biocultural scenario IV: biodiversity peaks are generated due to systematic deployment of H-EMs over time and space, increasing niche diversity and beta and gamma biodiversity. Species co-evolve and adapt to novel mosaic landscapes. The hypothesis predicts that historic biocultural landscapes will have greater biodiversity than ecosystems with intermediate disturbances as well as low-disturbance and degraded ecosystems.
The alternative biocultural hypothesis diverges from the IDH in that it does not operate on the premise of anthropogenic disturbances, but rather on the understanding of ‘mediations’, as previously discussed. According to the Royal Spanish Academy (RAE), the term ‘disturbance’ is derived from the Latin word dissentio (discord), referring to any event that disrupts the prior equilibrium between elements within a given context, thus serving as an antonym to ‘order’. In the context of the IDH, disturbances – whether low, intermediate or high – disrupt the natural order of ecosystems, with intermediate disturbances creating a ‘new order’ that is favourable to biodiversity. Despite years of accumulated evidence showing that certain human interventions in ecosystems do not necessarily lead to biodiversity loss, ecological theory continues to frame the outcome of increased biological diversity within the framework of the ‘disturbance paradox’ (Downey et al. Reference Downey, Walker, Moschler, Penados, Peterman, Pop and Song2023). In contrast, the biocultural hypothesis does not regard interventions as disruptions to ecosystemic order, but rather as a dialectical and historical process of landscape mediation between our species and its environment, rooted in the cognitive, labour, technological, organizational and symbolic characteristics of H. sapiens. Consequently, rather than focusing on cases where disturbance levels are intermediate, we propose that it is H-EMs – typically characterized by historical depth (through long processes of trial and error), broad geographical scale and cultural anchoring – that drives increases or even marked peaks in biodiversity.
The important underlying reasoning regarding succession is that biological species in historical biocultural landscapes are continuously and systematically subjected to H-EM, with a dynamic outcome founded on co-adaptive and creative environmental interrelationships. These H-EMs conditions adjust to source (high)–sink (low) dynamics of habitat quality (Dias Reference Dias1996), in which some keystone biocultural species (Lukawiecki et al. Reference Lukawiecki, Moola and Roth2024) respond very favourably to the newly created environmental conditions, providing the basis for other species to adapt to these environments through ecological interactions. To empirically elucidate this biocultural hypothesis, research needs to include sampling designs over large areas with consideration of palaeo-landscape, archaeological and ethnoecological studies to assess the historical depth of H-EMs. This will allow comparisons of species diversity in biocultural landscapes with reference, degraded and naturally or anthropogenic disturbed ecosystems (Fig. 2).
Implications: towards Priority Biocultural Areas
Our concept of ‘Priority Biocultural Areas’ is illustrated with global examples. Levis et al. (Reference Levis, Flores, Campos-Silva, Peroni, Staal and Padgurschi2024), for instance, identified cases in Brazil’s Amazon, Cerrado and Atlantic Forest regions described as ‘socio-ecological hopespots’. These regions express the biocultural particularity of having more than 1500 endemic species and a record of significant human management influence and maintenance of historic landscapes. In these areas, sustained collaborative partnerships between Indigenous knowledge and practices and scientific expertise have generated provocative research. These interdisciplinary programmes have fostered conservation efforts that focus on maintaining historically shaped landscapes with significant biocultural influence.
Similarly, Mabele et al. (Reference Mabele, Krauss and Kiwango2022) examined how the non-Western philosophy of Ubuntu – a principle rooted in ethical ecological values, emphasizing mutual care among humans, non-humans and the physical world – guides biocultural conservation frameworks in African countries, including Zimbabwe, Mozambique, Zambia, Malawi, Namibia and South Africa. These Indigenous frameworks do not adhere to conventional top-down protected area (PA) models. Instead, these models are based on the concept of ‘promoted areas’, where, rather than isolating biodiversity-rich environments from human societies, the conservation strategies are promoted through active use for, to and by knowledgeable humans.
An example of combining a PA with local community involvement is that of the Maya Forest at the ancient Maya centre of El Pilar in Belize and Guatemala (Ford & Ellis Topsey Reference Ford, St Clair, Archer, Brown, Barrett and Rodríguez2019). The binational management planning process is explicitly designed to engage citizen science in conserving the culture and nature of the site through biocultural integration. Recognition of the Maya flora and fauna as part of the ancient Maya landscape related to contemporary traditional land-use strategies brings to the fore the importance of citizen science in the development of priority biocultural conservation areas (Ford Reference Ford, St Clair, Archer, Brown, Barrett and Rodríguez2019). Furthermore, this initiative promotes among rural children and youth the learning of the complex historical environmental management practices carried out by their parents and grandparents. This is achieved through a practical forest garden and a biocultural pedagogical model referred to as ‘Känan K’aax’ (meaning ‘to care for the forest by managing it’ in Yucatec Maya).
Another example comes from the experience in Hawai‘i of the communities Hāʻena, Kaua‘i; Heʻeia, O‘ahu; and Ka‘ūpūlehu. Here, legal state and federal governmental authorities recognize Indigenous stewardship of territories of life. This recognition promotes community-driven maintenance of biocultural management schemes for their historical landscapes as the main conservation strategy (Winter et al. Reference Winter, Vaughan, Kurashima, Wann, Cadiz and Kawelo2023).
We argue that these landscape examples have been configured by H-EM processes. Validating our biocultural hypothesis on H-EMs has been implicitly recognized around the world; we call for explicit efforts. The examples we have discussed provide empirical support of our proposal that the causal relationships between humans and their environments are not simply biogeographical (cf. Steward Reference Steward1955); they are where biodiversity hotspots coincide with areas with high Indigenous and local rural populations (e.g., Clement et al. Reference Clement, Casas, Parra-Rondinel, Levis, Peroni and Hanazaki2021, Molnár et al. Reference Molnár, Fernández-Llamazares, Schunko, Teixidor-Toneu, Jarić, Díaz-Reviriego and Hill2023, Fraser et al. Reference Fraser, Cosiaux, Walters, Asiyanbi, Osei-Wusu and Addo-Fordjour2024).
The importance of prioritizing biocultural areas acknowledges the complex processes of human ecological learning and culturally bounded mechanisms that sustain landscape knowledge and management over time. Investigation of biocultural areas in regions across the globe requires concerted efforts that build collaborative research programmes. Transdisciplinary teams, led by Indigenous peoples and local rural communities, can complement biocultural understandings of the mechanisms and patterns that give rise to different levels of biodiversity. It is essential that these programmes incorporate the historical, institutional and symbolic management factors that contribute to the shaping of ecologically diverse biocultural landscapes (Ford & Nigh Reference Ford and Nigh2015, Walters et al. Reference Walters, Fraser, Picard, Hymas and Fairhead2019, Bray et al. Reference Bray, Hahn, Lourdusamy and Saraiva2023, Molnár et al. Reference Molnár, Fernández-Llamazares, Schunko, Teixidor-Toneu, Jarić, Díaz-Reviriego and Hill2023).
We see two main pathways to promote the institutionalization of Priority Biocultural Areas. First, there is governmental recognition of biocultural areas as Other Effective Area-Based Conservation Measures (OECMs), defined and promoted by Decision 14/8 of the Convention on Biological Diversity (CBD). Distinct from conventional PAs, OECMs are conservation models driven by the sociocultural values inherent to Indigenous and local contexts (Alves-Pinto et al. Reference Alves-Pinto, Geldmann, Jonas, Maioli, Balmford, Latawiec and Strassburg2021). Second, there is governmental recognition of self-managed biocultural conservation of historic territorial landscapes based on Indigenous peoples’ and rural local communities’ rights, responsibilities, values, governance systems and communal land access and use arrangements (IUCN 2010). Historic territories could be coordinated with Indigenous peoples and the Community Conserved Areas and Territories Consortium (ICCA). ICCA is a non-profit organization composed of more than 180 Indigenous organizations and more than 500 legal and academic advisors who lead collective actions at the local, national, regional and international levels to promote the defence and self-governance of the territories of life of Indigenous peoples and local communities (ICCA Consortium 2021). We can imagine scenarios where the peoples promoting historic territories of life could actively seek recognition as OECMs by their own countries.
Global research programmes prioritizing biocultural areas would significantly strengthen the engagement of Indigenous and local partners in their biodiversity-rich environments. Research programmes recognizing management partners will legitimize and institutionalize frameworks for Priority Biocultural Areas. A requisite is a thorough understanding the longue durée – the deep historical roots of biocultural landscapes. Incorporating local and Indigenous management practices and frameworks, as a contribution of citizen scientists, would fortify administrative, financial and decision-making processes. These frameworks are unique from yet comparable to the goals of conventional ecological approaches. Pursuing the biocultural hypothesis and inviting H-EMs into the equation would enable the integration of biodiversity conservation interests with local livelihoods and ontologies (Gavin et al. Reference Gavin, McCarter, Mead, Berkes, Stepp, Peterson and Tang2015, Caillon et al. Reference Caillon, Cullman, Verschuuren and Sterling2017). There could be recognition of the contribution of human interventions and testing of the H-EMs hypothesis as a critical subject of research that opens a world of possibilities at a time when embracing diversity is our challenge.
Acknowledgements
We thank CLACSO’s Political Agroecology Working Group.
Financial support
Pronaii Conahcyt 321286. DGAPA, UNAM, IN224023.
Competing interests
The authors declare none.
Ethical standards
Not applicable.







