Introduction
The neonatal gut microbiota rapidly acquires a diversity of bacteria from the mother, which then undergoes selection and succession as anaerobic conditions develop and oxygen is depleted (Hill et al., Reference Hill, Lynch, Murphy, Ulaszewska, Jeffery, O’Shea, Watkins, Dempsey, Mattivi and Tuohy2017; Ferretti et al., Reference Ferretti, Pasolli, Tett, Asnicar, Gorfer, Fedi, Armanini, Truong, Manara and Zolfo2018). Subsequently, Actinomycetota, such as Bifidobacterium, become more predominant from 1 week to 14 months of age, primarily reflecting the level of milk consumption and breastfeeding in comparison to infant formula feeding (Stewart et al., Reference Stewart, Ajami, O’Brien, Hutchinson, Smith, Wong, Ross, Lloyd, Doddapaneni and Metcalf2018). As infants transition to a higher proportion of solid foods and reduce their consumption of milk, their gut microbiota diversity increases remarkably between 15 and 30 months (Stewart et al., Reference Stewart, Ajami, O’Brien, Hutchinson, Smith, Wong, Ross, Lloyd, Doddapaneni and Metcalf2018).
This period is characterised by the emergence of additional genera belonging to the Bacteroidota phylum, which produce short-chain fatty acids (SCFAs), such as acetate, and members of the Bacillota phylum, such as Blautia from the Lachnospiraceae family, which produce acetate and butyrate. These SCFAs help maintain gut integrity, support immune function, and provide energy to colon cells (Holmberg et al., Reference Holmberg, Feeney, PK, Puértolas-Balint, Singh, Wongkuna, Zandbergen, Hauner, Brandl and Nieminen2024). By around 31 months, the microbiota diversity stabilises, marked by higher levels of Bacillota (e.g., Faecalibacterium and Roseburia) (Hill et al., Reference Hill, Lynch, Murphy, Ulaszewska, Jeffery, O’Shea, Watkins, Dempsey, Mattivi and Tuohy2017; Stewart et al., Reference Stewart, Ajami, O’Brien, Hutchinson, Smith, Wong, Ross, Lloyd, Doddapaneni and Metcalf2018).
Emerging evidence suggests that dietary transitions during infancy significantly influence the composition and diversity of the gut microbiota, thereby shaping host immune function, metabolism, and overall health outcomes through the acquisition of environmental microbes and the production of SCFAs via microbial fermentation of dietary fibres (Davis et al., Reference Davis, Dinsmoor, Wang and Donovan2020). Compared to the limited range of nutrients in breast milk, the introduction of early solid foods provides a broader array of nutrients, including diverse sources of fats, proteins, carbohydrates, and fibres, as well as exposure to additional microbes. These changes promote the diversification of the gut microbiota towards a more adult-like composition (Homann et al., Reference Homann, Rossel, Dizzell, Bervoets, Simioni, Li, Gunn, Surette, de Souza and Mommers2021). Dietary components serve as substrates for microbial fermentation in the gut, leading to the production of metabolites, including the SCFAs acetate, propionate, and butyrate. These SCFAs play essential roles in regulating host physiology, immune responses, and energy metabolism (Afzaal et al., Reference Afzaal, Saeed, Shah, Hussain, Rabail, Socol, Hassoun, Pateiro, Lorenzo and Rusu2022; Di Profio et al., Reference Di Profio, Magenes, Fiore, Agostinelli, La Mendola, Acunzo, Francavilla, Indrio, Bosetti and D’Auria2022). These dietary transitions, such as the reduction in milk consumption, the introduction of solid food, and the increased diversity of complex plant-derived carbohydrates during weaning, influence SCFA production through the succession of butyrate-producing microbes (Appert et al., Reference Appert, Garcia, Frei, Roduit, Constancias, Neuzil-Bunesova, Ferstl, Zhang, Akdis and Lauener2020). Although many host-related factors are best investigated through in vivo studies, in vitro models are more suitable for determining the impact of specific nutritional interventions in each section of the gastrointestinal tract (Van den Abbeele et al., Reference Van den Abbeele, Duysburgh, Cleenwerck, Albers, Marzorati and Mercenier2021), in the absence of environmental sources of microbes.
The aim of this study was to determine the rate of shift in the infant gut microbiota composition and the resulting metabolite balance during a dietary transition representing the reduction of milk consumption and an increase in complex carbohydrates. By using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME), the effects of the host immune system, absorption of SCFAs, and other host-dependent factors can be subtracted (Van den Abbeele et al., Reference Van den Abbeele, Duysburgh, Cleenwerck, Albers, Marzorati and Mercenier2021) to reveal the process of adaptation of the endogenous microbial community in response to the consecutive conditions of the colon.
Methods
Twin SHIME model
The Twin SHIME system was set up, adapted, validated, and operated in accordance with the SHIME manual (ProDigest-Ghent University, Belgium). The Twin SHIME system consisted of 10 vessels, configured as 2 parallel series of 5 vessels (Figure 1), each encompassing a stomach, a small intestine, and 3 colon regions: ascending colon (AC) (volume = 500 mL, pH = 5.6–5.9), transverse colon (TC) (volume = 800 mL, pH = 6.15–6.4), and descending colon (DC) (volume = 600 mL, pH = 6.6–6.9). The temperature was controlled at 37°C using a water bath, and the pH was adjusted in each colon vessel with 0.5 M NaOH or HCl. In addition, the vessels were continuously stirred at 300 rpm for a duration of 3 weeks. SHIME 1 and 2 served as replicates receiving the exact same treatments (Van den Abbeele et al., Reference Van den Abbeele, Roos, Eeckhaut, MacKenzie, Derde, Verstraete, Marzorati, Possemiers, Vanhoecke and Van Immerseel2012).
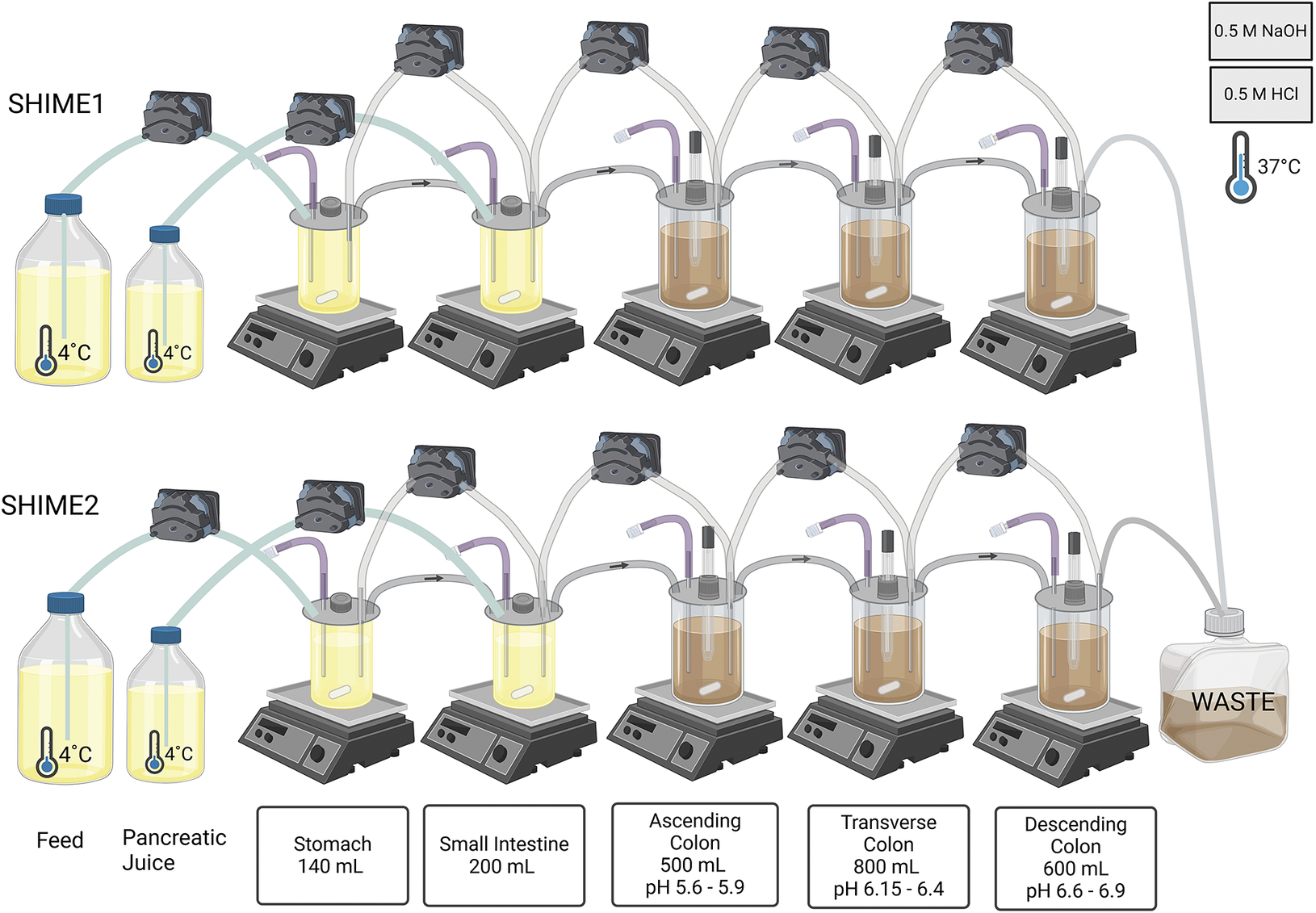
Figure 1. Overview of the Twin SHIME® system utilised in the study showing the setup, and configuration of the SHIME® system, including the compartments and associated conditions used for simulating the human gastrointestinal tract. In this study, SHIME® 1 and SHIME® 2 functioned as replicates undergoing the same series of treatments.
Faecal sample collection, slurry preparation, and inoculation
An infant donor was selected based on specific criteria to ensure a controlled and consistent baseline microbiota for this study (Mesnage et al., Reference Mesnage, Calatayud, Duysburgh, Marzorati and Antoniou2022). These criteria include an infant between 10 and 12 months of age who had not been introduced to solid foods and was exclusively fed breast milk and formula with a healthy infant microbiota composition, dominated by Actinomycetota, Bacillota, and Bacteroidota, which are the most abundant phyla in infants at this developmental stage (Jost et al., Reference Jost, Lacroix, Braegger, Rochat and Chassard2014; Hill et al., Reference Hill, Lynch, Murphy, Ulaszewska, Jeffery, O’Shea, Watkins, Dempsey, Mattivi and Tuohy2017; Derrien et al., Reference Derrien, Alvarez and de Vos2019). The selected donor was a vaginally born, healthy, 11-month-old male who fit these criteria and had not received antibiotics for at least 3 months before sampling. Faecal samples were aseptically collected using sterilised containers. Subsequently, 20 g of the faecal sample was homogenised in 100 mL of 0.1 M phosphate buffer (20% w/v) using a stomacher at 200 rpm for 30 s (Deyaert et al., Reference Deyaert, Moens, Pirovano, van den Bogert, Klaassens, Marzorati, Van de Wiele, Kleerebezem and Van den Abbeele2023). The resulting suspension was aliquoted into two 50 mL Falcon tubes and centrifuged at 500×g for 2 min. The supernatant was collected to obtain the final suspension. Each colon vessel was inoculated with faecal slurry at 5% AC (500 mL with 25 mL of inoculum), TC (800 mL with 40 mL of inoculum), and DC (600 mL with 30 mL of inoculum). Immediately after inoculation, automatic pH control was initiated, and the culture was allowed to incubate overnight before the initiation of the feeding schedule for the 7-day stabilisation period and then the 10-day treatment period.
Feed and pancreatic juice preparation and uptake
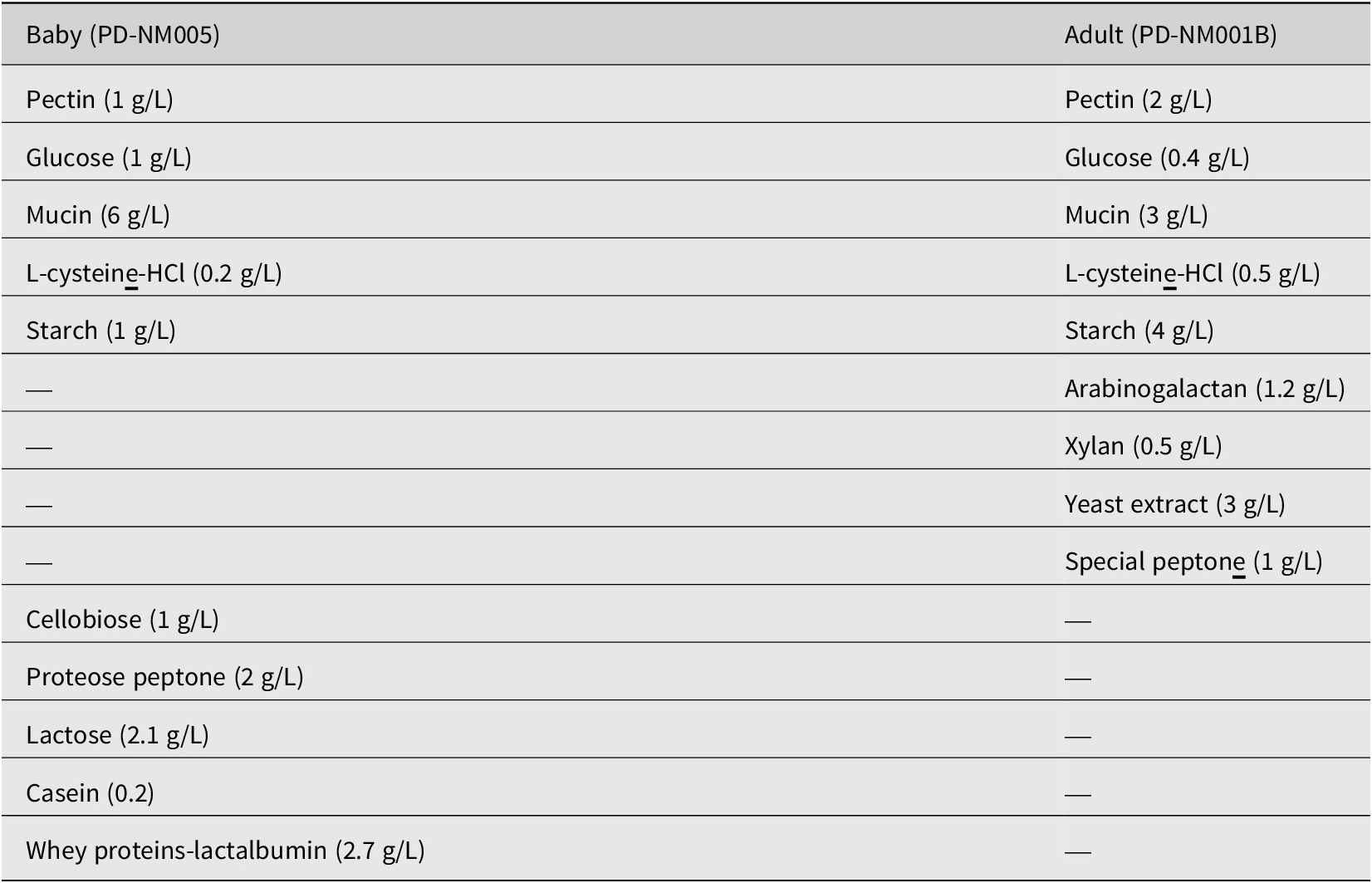
To prepare the feed, 17.2 g/L of medium (PD-NM005) for baby feed and 15.6 g/L of L-SHIME medium (PD-NM001B) for adult feed were added to 1 L of distilled water (Table 1). After thorough mixing, the medium was autoclaved, and the pH was adjusted according to the ProDigest guidelines. The resulting feed was stored at 4°C and continuously stirred at 300 rpm throughout the entire SHIME run. The feed volume was 140 mL three times a day at 8-h intervals, with pumping initiated at 5:00 PM, 1:00 AM, and 9:00 AM into the two stomach vessels. The pancreatic juice was prepared by dissolving NaHCO3 (12.5 g/L, Anachemia, VWR International, 470302-440); Ox bile (6 g/L, Difco™, BD Biosciences, DF0128-17-8); and pancreatin (0.9 g/L, Alfa Aesar, Thermo Scientific, J19880.28) in distilled water, using a heater for thorough mixing. The resulting solution was stored at 4°C and continuously stirred at 300 rpm throughout the entire SHIME run. Pancreatic juice was added to the small intestine vessels at a concentration of 60 mL per feeding session three times a day. Pumping was initiated at 6:30 PM, 2:30 AM, and 10:30 AM. At the end of the 10-day feed treatment period, luminal samples were collected from AC1, AC2, TC1, TC2, DC1, and DC2 for DNA extraction, 16S ribosomal RNA (rRNA) gene amplicon sequencing, and SCFA analysis.
Table 1. Composition of the baby and adult feeds used in the experiment
DNA extraction and 16S rRNA gene amplicon sequencing
Total volume of 1 mL of luminal samples was centrifuged at (10,000×g, 10 min, 4°C) to collect cell pellets. Subsequently, DNA extraction was carried out using the DNeasy PowerSoil Pro Kits (Qiagen, MD, USA) following the manufacturer’s protocol. The quantity and quality of the extracted DNA were assessed through fluorometry (Qubit 4, Invitrogen, Waltham, MA, USA) and spectrometry (NanoDrop 1000; Thermo Scientific, Waltham, MA, USA), respectively. The DNA samples underwent dilution to 10 ng/mL and were subsequently submitted to the Advanced Analysis Centre (AAC) at the University of Guelph, Guelph, Ontario, Canada, for sequencing of the V3–V4 regions of the 16S rRNA gene amplicon using the Illumina MiSeq platform (Illumina, San Diego, CA, USA). Amplification of the V3 and V4 regions (~460 bp) of the 16S rRNA gene was achieved utilising the following universal primers: forward primer: 5′-CCTACGGGNGGCWGCAG-3′ and reverse primer: 5′-GACTACHVGGGTATCTAATCC-3′ (Klindworth et al., Reference Klindworth, Pruesse, Schweer, Peplies, Quast, Horn and Glöckner2013). Raw sequence reads underwent filtering using the Miseq Sequencer System software to eliminate low-quality sequences and were trimmed to eliminate adapter sequences. The resultant reads ranged up to 301 bases in length.
SCFA quantification by 1H-nuclear magnetic resonance
Then, 5 mL of luminal samples were subjected to filtration using a double-layer polystyrene filtration system in two steps: first with a 0.84-μm pore size filter, then with a 0.22-μm pore size filter, and subsequently stored at –20°C. Following thawing, 540 μL of the filtered luminal samples were mixed with 60 μL of IS-2 Chenomx Internal Standard-DSS-d6 (deuterated 2,2-dimethyl-2-silapentane-5-sulfonate-d6 sodium salt (5 mM) and sodium azide (0.2% w/v) in 99.9% D2O) (Chenomx, 613150). This resulting mixture was then carefully transferred to a 5-mm thin-wall precision nuclear magnetic resonance (NMR) sample tube (Wilmad-LabGlass, NJ, USA, WG-1000-4) and submitted for analysis at the NMR Centre of the AAC (University of Guelph, Guelph, ON).
Bioinformatics and data analysis
All sequence data were processed using the DADA2 pipeline through the MicrobiomeAnalyst 2.0 server (Lu et al., Reference Lu, Zhou, Ewald, Pang, Shiri and Xia2023b). This process encompassed filtering, denoising, merging read pairs, and the removal of chimaeras. Finally, the operational taxonomic units (OTUs) were generated based on the SILVA database version 138.1 (McLaren and Callahan, Reference McLaren and Callahan2021). Two samples, one from an adult-fed vessel (AC1) and one from a baby vessel (AC1), were removed due to insufficient read counts, notably falling below 500 reads. The statistical analysis was performed on the MicrobiomeAnalyst 2.0 server. Sequences with counts lower than 4 and a prevalence below 20% were filtered out. Following this, data normalisation was performed using total sum scaling, and the data were subsequently rarefied to a minimum library size of 40,662 sequences. This step aimed to alleviate any potential biases arising from differences in sequencing depth among various samples. Alpha diversity was assessed using the Shannon index, comparing the adult-fed and baby-fed groups, as well as among the AC, TC, and DC groups, using Welch’s t-test for pairwise comparisons and analysis of variance (ANOVA) for multiple group comparisons. For β-diversity analysis, principal coordinate analysis with the Bray–Curtis index was employed, followed by permutational multivariate ANOVA (PERMANOVA) to determine statistical significance among the groups. Principal component analysis (PCA) was conducted on microbial compositional data, including all genera above the filtering threshold, after Z-score normalisation to visualise shifts in microbial communities across feeds for the AC, TC, and DC vessels. Linear discriminant analysis effect size (LEfSe) was then used to detect significant differences in bacterial abundance between the treatment groups.
Results
The baby and adult feeds differed in terms of simple versus complex carbohydrates and the presence or absence of bovine milk proteins (Table 1). The baby feed, which was intended to simulate the diet of post-weaning infants, contained a higher level of glucose (1 g/L) and lactose (2.1 g/L), while the adult feed had higher concentrations of starch (4 g/L) and pectin (2 g/L), as well as arabinogalactan and xylan. In addition, the baby feed includes casein (0.2 g/L) and whey proteins (2.7 g/L), which were not present in the adult feed. Proteins were present in both feeds, with the baby feed containing proteose peptone (2 g/L), casein, and whey, whereas the adult feed included special peptone (1 g/L) and yeast extract (3 g/L). The total carbohydrates in the baby feed amounted to 6.1 g/L, compared to 8.1 g/L in the adult feed. Conversely, the total protein content was slightly higher in the baby feed at 4.9 g/L compared to 4 g/L in the adult feed. As both feed types were autoclaved, no additional microbes were introduced into the stabilised microbial community over the period of study.
The two parallel SHIME systems, each comprising a stomach, small intestine, and three colon regions (AC, TC, and DC), were operated for 1 week to allow a sufficient period of adaptation before starting the feed transition. Microbiota stabilisation was confirmed by measuring butyrate, propionate, and acetate levels on 2 separate days, spaced 3 days apart, during the initial 1-week period of baby feed administration. Consistent SCFA production over two sequential measurements confirmed the stability of the metabolic activity of the microbiota (Van de Wiele et al., Reference Van de Wiele, Van den Abbeele, Ossieur, Possemiers and Marzorati2015).
A total of 1,160,868 high-quality reads with an average read count of 105,553 per sample were obtained after removing low-quality and chimeric sequences. The examination of rarefaction curves alongside an average Good’s coverage index of 99.99 ± 0.001 suggests that the sequencing depth comprehensively captured the bacterial diversity present in all samples examined in this study. With the OTU table (Supplementary Material S1), 1571 OTUs were considered for analysis after removing features with a minimum count below 4 and those with <10% variance across samples. In summary, within the baby-fed group, Bacillota was the most prevalent phylum at 37.7%, followed by Actinomycetota at 33.7% and Bacteroidota at 26.3%. In the adult-fed group, Actinomycetota emerged as the predominant phylum at 32.6%, followed by Pseudomonadota at 24.6% and Bacteroidota at 23.1%.
The alpha diversity did not differ significantly between the adult-fed and baby-fed treatments, as measured with the Shannon index, which considers both species richness and evenness (p-value: 0.102). The beta diversity analysis, utilising the Bray–Curtis similarity index, with PERMANOVA as the statistical method, revealed significant differences (p-value: 0.008) in microbial diversity between the baby-fed and adult-fed vessels (Figure 2A). PCA revealed a clear shift in microbial composition between baby-fed and adult-fed conditions across AC, TC, and DC colon sites (Figure 2B). This shift was consistent in direction across the AC, TC, and DC, highlighting a structured microbial transition within the conditions of the three vessels. Bifidobacterium was found to be the predominant genus in both baby-fed and adult-fed groups across all colon sites (AC, TC, and DC) (Figure 3A). LEfSe analysis revealed significant differences in genera among the treatment groups in all three colon sites (Figure 3B). Following the transition to adult feed, several genera were significantly reduced, including Blautia, Parabacteroides, Megasphaera, and Flavonifractor. Conversely, other genera, such as Escherichia–Shigella, Klebsiella, Bacteroides, Akkermansia, Enterobacter, Citrobacter, Clostridium, Lactobacillus, Yokenella, and Veillonella, were significantly higher in the adult-fed vessels.
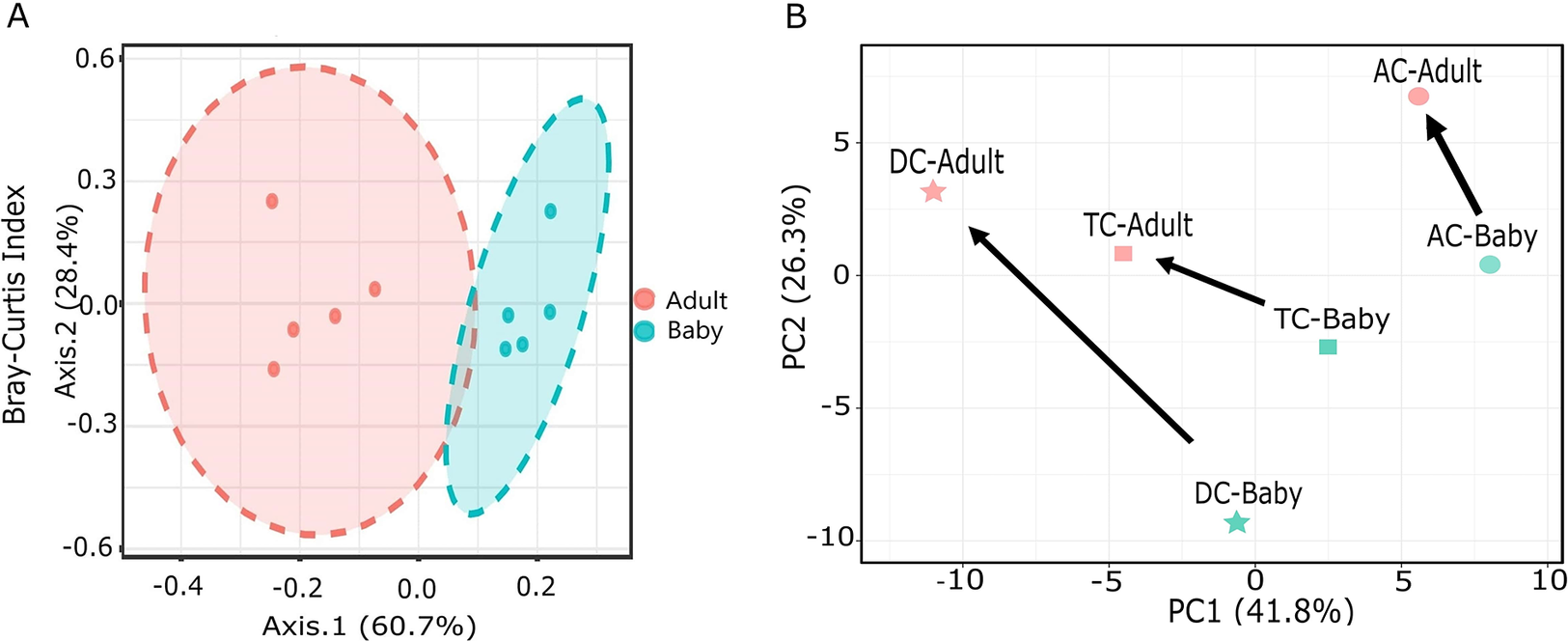
Figure 2. (A) Principal coordinate analysis (PCoA) based on the Bray–Curtis similarity index, illustrating beta diversity differences between baby and adult feeds. (B) Principal component analysis (PCA) illustrating microbial compositional shifts, with arrows indicating the transition from baby to adult feeding for each vessel.

Figure 3. (A) Relative abundance of bacteria at the genus level among the feed types and colon sites. (B) Linear discriminant analysis effect size (LEfSe) at the genus level between the feed types. An LDA score > 2 was used to determine significantly different genera between the groups.
Regarding metabolic activity, the amount of total SCFA was significantly different between baby feed and adult feed treatments only for the TC, but not for the AC or DC regions (Figure 4). No significant differences were detected in acetate and propionate amounts or molar ratios between baby-fed and adult-fed vessels across all colon sites (Figures 4 and 5). However, a significant reduction in butyrate amount and molar ratio was observed after transitioning from baby feed to adult feed in both the TC and DC regions (two-tailed t-test, p-value < 0.05), which was not observed in the AC vessel.

Figure 4. Concentration of acetate, butyrate, propionate, and total SCFA in the AC, TC, and DC vessels of the TwinSHIME system fed with baby and adult feed.
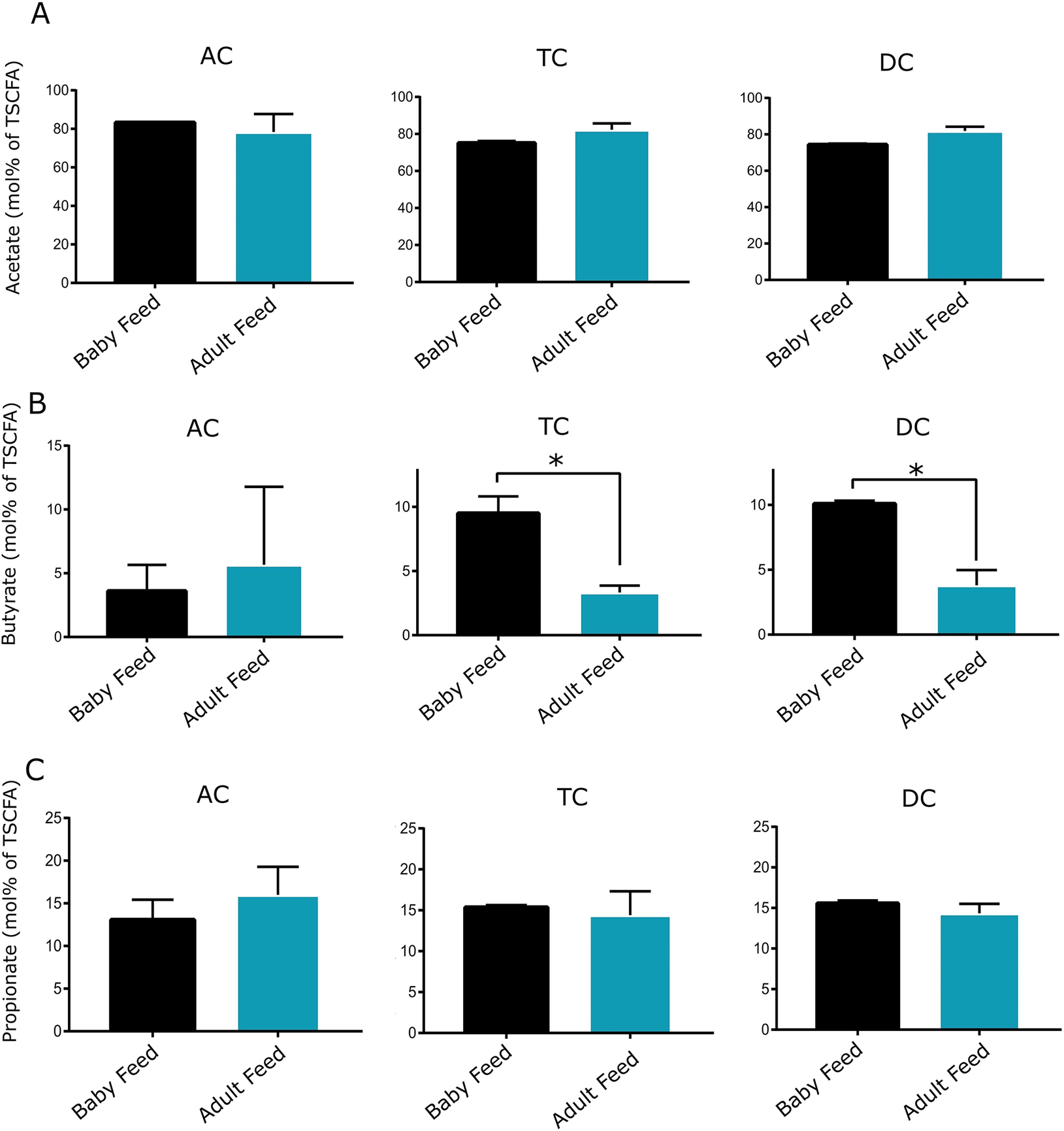
Figure 5. Molar proportion of acetate, butyrate, and propionate in the AC, TC, and DC vessels of the TwinSHIME system fed with baby and adult feed.
Discussion
In this study, the Twin SHIME® system was used to correlate changes in the gut microbiota composition with metabolic activity during the transition from baby feed to adult feed in conditions mimicking the three sections of the colon. The SHIME was employed to simulate infant gut microbiota under standardised conditions, minimising interindividual differences caused by variations in diet, pH, transit time, and other factors (Šuligoj et al., Reference Šuligoj, Vigsnæs, Van den Abbeele, Apostolou, Karalis, Savva, McConnell and Juge2020). Over the stabilisation period, the SHIME establishes a steady-state microbial community, controlled by reproducible factors, such as feed content, pH, enzymes, and transit time (Šuligoj et al., Reference Šuligoj, Vigsnæs, Van den Abbeele, Apostolou, Karalis, Savva, McConnell and Juge2020). This model also allows for a longitudinal study design, enabling the comparison of treatment effects with both the initial baseline and a concurrently run control (Van den Abbeele et al., Reference Van den Abbeele, Duysburgh, Cleenwerck, Albers, Marzorati and Mercenier2021).
The higher content of simple carbohydrates, including glucose and lactose, in the baby feed supports the growth of Bacillota populations, which primarily metabolise these easily fermentable carbohydrates (Gong et al., Reference Gong, Zhou, Luo, Gesang and Suolang2020). Specifically, Bacillota contribute significantly to the gene repertoire of glycoside hydrolases and carbohydrate esterases (Gong et al., Reference Gong, Zhou, Luo, Gesang and Suolang2020). Bacillota play a role in the human gut by fermenting carbohydrates, such as glucose and lactose, to produce SCFAs, such as acetate, propionate, and butyrate, which are important for gut health and energy metabolism (Desai and Landay, Reference Desai and Landay2018; Baltazar-Díaz et al., Reference Baltazar-Díaz, Andrade-Villanueva, Sánchez-Álvarez, Amador-Lara, Holguín-Aguirre, Sánchez-Reyes, Álvarez-Zavala, López-Roa, Bueno-Topete and González-Hernández2024).
Blautia, Parabacteroides, and Megasphaera genera were more abundant in the microbial communities under the baby feed. Studies have shown that Blautia species thrive in environments rich in short-chain fructooligosaccharides (Mao et al., Reference Mao, Gu, Li, Cui, Zhao, Zhang and Chen2018) and lactulose (Cui et al., Reference Cui, Gu, Liu, Li, Mao, Zhang, Zhao and Chen2021), and are known for producing butyrate via the acetyl-CoA pathway (Zhou et al., Reference Zhou, Pan, Xin, Zhang, He, Chen, Liu, Chen and Fan2017). Furthermore, lactose, a major carbohydrate in ProDigest baby feed, supports the growth of bacteria such as Bifidobacterium that are well-known for their ability to metabolise this disaccharide. The lactate produced from lactose or xylose fermentation by these bacteria can then be utilised by Megasphaera, which converts lactate into butyrate, which is an SCFA essential for maintaining gut health (Zhao et al., Reference Zhao, Lau, Zhong and Chen2024). This cross-feeding interaction provides Megasphaera with a competitive advantage by allowing it to increase in environments where lactose or xylose are present, thereby contributing to a beneficial gut environment (Shetty et al., Reference Shetty, Marathe, Lanjekar, Ranade and Shouche2013). Previous research has shown that a lower abundance of Megasphaera as a biomarker of gut health in infants is associated with an increased risk of diarrheal symptoms in cryptosporidiosis (Carey et al., Reference Carey, Medlock, Alam, Kabir, Uddin, Nayak, Papin, Faruque, Haque and Petri2021). The genus Parabacteroides includes several species with a high number of mucin-degrading carbohydrate-active gene clusters per genome, which facilitates their proliferation in environments containing mucin (Pruss et al., Reference Pruss, Marcobal, Southwick, Dahan, Smits, Ferreyra, Higginbottom, Sonnenburg, Kashyap and Choudhury2021). In our study, the baby feed contained a higher mucin concentration of 6 g/L compared to the adult feed, which contained 3 g/L mucin. This higher mucin content in the baby feed provided a favourable substrate for the growth of Parabacteroides strains, which are specialised in degrading mucin effectively (Pan et al., Reference Pan, Barua and Ip2022). These mucin-degrading gut commensals, particularly Parabacteroides spp., exhibit anti-inflammatory properties and enhance the epithelial barrier (Pan et al., Reference Pan, Barua and Ip2022). Akkermansia is also known for its ability to degrade mucin and is supported by the presence of mucin in both baby and adult feeds (Fricker et al., Reference Fricker, Yao, Lindemann and Flores2024). Although the mucin concentration in the baby feed was higher (6 g/L) compared to the adult feed (3 g/L), Akkermansia showed increased abundance during the adult feed period. This suggests that other factors, such as cross-feeding from complex carbohydrates or proteins present in the adult feed, may have contributed to the higher relative abundance of Akkermansia, indicating that the metabolism of the complex carbohydrates in the adult feed created a favourable environment for its growth (Belzer and De Vos, Reference Belzer and De Vos2012). The combination of Bifidobacterium and prebiotic fibres has been shown to promote the abundance of Akkermansia (Zhang et al., Reference Zhang, Hu, Tan, Zhong and Nie2022b).
The adult feed contains a higher concentration of complex carbohydrates, such as pectin, starch, arabinogalactan, and xylan. Bacteroidota and Actinomycetota contribute to the digestion of starch and complex polysaccharides, such as pectin, arabinogalactan, and xylan, breaking them down into SCFAs, such as acetate and propionate that further support gut function (Vinke et al., Reference Vinke, Aidy and Van Dijk2017; Fujita et al., Reference Fujita, Sasaki and Kitahara2019; Rinninella et al., Reference Rinninella, Raoul, Cintoni, Franceschi, Miggiano, Gasbarrini and Mele2019). Typically, Bacteroides produces more acetate than propionate. In our study, acetate was dominant in the overall SCFA profile observed, coinciding with the higher abundance of Escherichia–Shigella and Bacteroides (Cockburn and Koropatkin, Reference Cockburn and Koropatkin2016; Zhang et al., Reference Zhang, Fan, Huang and Zuo2022a). However, neither the amount nor the molar proportion of acetate differed between the two feeds. The presence of both fibre-degrading and carbohydrate-utilising bacteria determines the equilibrium of SCFA metabolites (Flint et al., Reference Flint, Scott, Duncan, Louis and Forano2012). Bifidobacterium was found in both baby-fed and adult-fed groups across all colon sites. Bifidobacterial species tend to dominate the intestines of healthy breastfed infants, but the dominant species change with age, generally decreasing in adulthood and stabilising at lower levels (Arboleya et al., Reference Arboleya, Watkins, Stanton and Ross2016), while its abundance can vary significantly between individuals (Lu et al., Reference Lu, Zhang, Zhang, Chen, Zhao, Chen, Lu and Li2023a). The presence of lactose in baby feed explains the higher abundance of bifidobacteria observed during the baby feed period. As the diet transitions from baby feed to more complex adult feed, the presence of Bifidobacterium reflects the ability to degrade arabinogalactan and xylan substrates, producing acetate. This suggests that Bifidobacterium can be carried over into the adult period, maintaining acetate production in the absence of lactose, but at a lower relative abundance.
The stability of taxa such as Actinomycetota, which showed minimal changes in relative abundance between the baby-fed and adult-fed periods, shows the resilience of certain microbial populations during dietary shifts. Despite the dietary shift towards complex carbohydrates, which supports the proliferation of Bacteroides, Bifidobacterium maintains a stable presence, partly due to syntrophic interactions with Bacteroides (Munoz et al., Reference Munoz, James, Bottacini and Van Sinderen2020). These interactions are particularly evident in the degradation of arabinogalactan, a complex polysaccharide, where Bacteroides initially break down the polysaccharide into oligosaccharides and other intermediates, some of which are released into the surrounding environment. Bifidobacterium can then utilise these partially degraded products through cross-feeding, thus establishing a cooperative metabolic network (Munoz et al., Reference Munoz, James, Bottacini and Van Sinderen2020). However, the transition from a baby feed rich in simple sugars and oligosaccharides to an adult feed containing more complex carbohydrates raises the possibility that Bifidobacterium populations may decline over time due to the reduced availability of their preferred substrates (Odamaki et al., Reference Odamaki, Kato, Sugahara, Hashikura, Takahashi, Xiao, Abe and Osawa2016). Given that Bifidobacterium prefer simpler sugars, such as lactose, and are also adept at transporting and utilising a variety of oligosaccharides, including human milk oligosaccharides and prebiotics, their abundance may eventually decline in the absence of adequate fermentable substrates. These substrates are more prominent in baby diets, which could explain why Bifidobacterium populations may be less stable as the diet shifts to complex carbohydrates in adulthood. The timing of this decline could depend on how quickly other microbial populations, such as Bacteroides, dominate the ecological niche as they are better equipped to utilise the more complex carbohydrates present in the adult feed (Xiao et al., Reference Xiao, Zhang, Duan, Narbad, Zhao, Chen, Zhai, Yu and Tian2024).
Escherichia–Shigella were more abundant after the adult feed treatment, which was enriched in complex carbohydrates. Escherichia–Shigella, including commensal Escherichia coli species within the Pseudomonadota phylum, can utilise a range of monosaccharides and disaccharides derived from the breakdown of carbohydrates, producing acetate (Fabich et al., Reference Fabich, Jones, Chowdhury, Cernosek, Anderson, Smalley, McHargue, Hightower, Smith and Autieri2008). The Escherichia genus belongs to the Proteobacteria (Pseudomonadota) phylum and was found to increase in abundance during the transitional phase of the gut microbiota development of infants from 15 to 30 months of age (Stewart et al., Reference Stewart, Ajami, O’Brien, Hutchinson, Smith, Wong, Ross, Lloyd, Doddapaneni and Metcalf2018).
The milk proteins in baby feed, including both whey protein and casein, play a role in promoting Bacillota and Actinomycetota (Monteiro et al., Reference Monteiro, Roquetto, de Pace, Moura, Santos, Yamada, Saad and Amaya-Farfan2016; Beaumont et al., Reference Beaumont, Portune, Steuer, Lan, Cerrudo, Audebert, Dumont, Mancano, Khodorova and Andriamihaja2017). Whey proteins have been observed to support the growth of Bifidobacterium, which belongs to the phylum Actinomycetota (Petschow and Talbott, Reference Petschow and Talbott1990). Other bacteria also utilise whey proteins; however, studies indicate that whey protein more effectively stimulates the growth of Bifidobacterium compared to other protein sources, which is likely due to the specific adaptability of these bacteria to whey proteins, as they possess specialised enzymatic pathways, such as protease activity and peptide transport systems that enable them to efficiently metabolise whey proteins into acetate and lactate through amino acid-to-SCFA conversion pathways (Pescuma et al., Reference Pescuma, Hébert, Mozzi and De Valdez2010; Sánchez-Moya et al., Reference Sánchez-Moya, López-Nicolás, Planes, González-Bermúdez, Ros-Berruezo and Frontela-Saseta2017; Boscaini et al., Reference Boscaini, Skuse, Nilaweera, Cryan and Cotter2023). The acetate and lactate produced can be further utilised by other gut bacteria, particularly butyrate-producing bacteria, through cross-feeding interactions, influencing butyrate production in the gut (Zhao et al., Reference Zhao, Lau, Zhong and Chen2024). Some clostridia can convert amino acids such as glutamate to butyrate (Louis and Flint, Reference Louis and Flint2017). In contrast to the protein sources found in baby feed, the adult feed used in the study incorporates peptones derived from animal and plant proteins (Table 1). The consumption of animal proteins has been associated with an increase in the abundance of Pseudomonadota in the gut microbiota (Zhu et al., Reference Zhu, Lin, Zhao, Shi, Li, Li, Zhu, Xu, Li and Zhou2015). In one study, rats fed meat proteins exhibited a higher prevalence of the phylum Pseudomonadota compared to those fed casein, while the group fed soy protein demonstrated a significant increase in Bacteroidota (Zhu et al., Reference Zhu, Lin, Zhao, Shi, Li, Li, Zhu, Xu, Li and Zhou2015). This finding aligns with the notable rise in the genera Escherichia–Shigella, Klebsiella, Citrobacter, Enterobacter, and Bacteroides observed in our adult feed group. Interestingly, the same study also reported an increase in the abundance of the genus Lactobacillus among those consuming animal proteins, especially chicken and fish, compared to the casein group (Zhu et al., Reference Zhu, Lin, Zhao, Shi, Li, Li, Zhu, Xu, Li and Zhou2015).
Acetate and propionate levels did not differ between the baby- and adult-fed treatments across all colon sites, even though the starch and complex carbohydrate levels were higher during the adult feeding period. Thus, the change in carbon substrates between feeds did not modify the relative proportions of acetate and propionate-producing bacteria, even though the specific groups of microbes were altered in abundance. However, both the amount and the molar ratio of butyrate declined in the TC and DC regions after transitioning to adult feed. Elevated relative abundance of Escherichia–Shigella is often associated with a lower abundance of SCFA-producing bacteria (Hu et al., Reference Hu, Cheng, Yao, Lin, Li, Wang, Weng, Zou, Zhu and Zhi2022). This reduction in butyrate levels upon switching to adult feed could be attributed to the relative proportions of bacteria able to degrade arabinogalactan and xylan. Acetate-producing Blautia spp. can utilise starch but may not be able to degrade xylan (Chen et al., Reference Chen, Liu, Imam, Sun, Wang, Gu, Wen and Xin2020) or arabinogalactan (Nie et al., Reference Nie, Sun, Li, Zuo, Chen, Lin and Nie2023), explaining its decline under the adult feed. The stabilised microbiota composition in the SHIME model derived from the infant donor showed the presence of butyrate-producing bacteria, such as Lachnospiraceae, Ruminococcaceae, and Clostridiaceae. Megasphaera, which produces butyrate from organic acids, such as lactate, declined after the transition to adult feed but was maintained at a lower relative abundance. With this decline in both Blautia and Megasphaera, the combined relative abundance of minor components of the microbial community increased, such as Clostridium spp., which is considered one of the pioneering butyrate producers in the gut, but they produce low amounts of butyrate (Appert et al., Reference Appert, Garcia, Frei, Roduit, Constancias, Neuzil-Bunesova, Ferstl, Zhang, Akdis and Lauener2020). Appert et al. (Reference Appert, Garcia, Frei, Roduit, Constancias, Neuzil-Bunesova, Ferstl, Zhang, Akdis and Lauener2020) have demonstrated that butyrate-producing groups are generally absent in infant faeces but increase in prevalence and abundance over the first year of life. Some of these species have limited ability to use complex carbohydrates, such as Anaerobutyricum halli, so they depend on cross-feeding, and converting lactate and acetate to butyrate. Many Lachnospiraceae can utilise a diversity of carbohydrates, generating larger amounts of butyrate (Appert et al., Reference Appert, Garcia, Frei, Roduit, Constancias, Neuzil-Bunesova, Ferstl, Zhang, Akdis and Lauener2020). This implies that the relative ratio of Lachnospiraceae, Ruminococcaceae, Erysipelotrichaceae, and Clostridiaceae within the Bacillota group determines the rate of butyrate production. The decrease in butyrate in the adult-fed vessels is consistent with the decline in Lachnospiraceae (higher butyrate producers) and the significant increase in Clostridium (lower butyrate producers). As both the baby and adult feeds used in this protocol were sterilised, no additional source of environmental microbes was provided. This ensured that the contribution from the feed itself was eliminated, revealing the metabolic adaptability of the original microbial community from the donor. This result demonstrates how the environmental microbes consumed by infants after weaning contribute to the further development of the ability of the gut microbiota to metabolise complex carbohydrates and increase butyrate production over the first year of life (Appert et al., Reference Appert, Garcia, Frei, Roduit, Constancias, Neuzil-Bunesova, Ferstl, Zhang, Akdis and Lauener2020).
Akkermansia, a mucin-degrading bacterium, was more abundant in the adult feed group and is known to produce both propionate and acetate. In our study, the reduction in butyrate levels could be attributed to the fact that the 10-day transition period may not have been long enough for butyrate-producing bacteria to fully adapt and establish themselves if they were initially present at low levels (Zhao et al., Reference Zhao, Zhang, Ding, Wu, Lam, Wang, Fu, Xue, Lu and Ma2018). The SHIME system did not receive any exogenous microbes from feed, as would occur with a human infant. Complex carbohydrates can be fermented by a range of bacteria, some of which produce acetate or propionate rather than butyrate (Flint et al., Reference Flint, Scott, Duncan, Louis and Forano2012). Therefore, without sufficient butyrate-producing bacteria, the increased availability of complex carbohydrates might not result in higher butyrate production as expected (Cronin et al., Reference Cronin, Joyce, O’Toole and O’Connor2021). While the modulation of SCFA production is well-documented, our findings suggest that during the transition to adult feed, if the prevalence of fibre-degrading bacteria is insufficient, butyrate production can decrease. As the feed was sterilised, the introduction of additional fibre-degrading microbes was limited, which may have inhibited or delayed the full development of the adult microbiota profile. The combination of an adult feed containing a defined set of carbohydrates and the lack of exogenous incoming bacteria limited the further diversification of the gut microbiota. This highlights the potential need for unsterilised feed or targeted microbial supplementation to reproduce the normal maturation of the microbial community to be able to efficiently produce butyrate.
As the concentration and percentage of acetate and propionate remained unchanged across the dietary transition, the functional capability of the gut microbiota was preserved despite changes in microbial taxa composition, supporting the resilience of the gut microbiota to this dietary transition. This suggests that dietary interventions focusing on promoting butyrate-producing bacteria may be advantageous to promote SCFA production during transitions such as weaning. The presence of adaptable bacterial taxa such as Bacteroides, Escherichia, and Akkermansia, contributes to the resilience of the gut microbiota, which can compensate for changes in other microbial populations while maintaining acetate and propionate production under varying dietary conditions (Wexler, Reference Wexler2007; Belzer and De Vos, Reference Belzer and De Vos2012; Lozupone et al., Reference Lozupone, Stombaugh, Gordon, Jansson and Knight2012).
Our study showed the shifts in microbial species and some concomitant changes in SCFA production patterns that can be associated with the replacement of simple sugars with complex carbohydrates and the switch from milk proteins to meat and plant proteins representing the transition from a baby to adult feed. Notably, the significant reduction in butyrate production following the switch to adult feed illustrates the importance of the diet in providing additional fibre-degrading microbes to maintain butyrate production after the shift from simple sugars to complex carbohydrates. These findings suggest that maintaining or enhancing the abundance of butyrate producers during dietary transitions could be important for sustaining optimal energy extraction during development. While this study provides valuable insights into microbial shifts during dietary transitions, the findings are based on a single donor and should be interpreted within the specific context of this controlled study. The dietary intervention demonstrated the capacity of certain bacteria from the infant gut microbiota, such as Akkermansia and Bacteroides, to adapt to adult feed, but future interventions may need to include targeted probiotics, fibres, or prebiotics that specifically promote the growth of butyrate-producing bacteria to restore and enhance butyrate levels.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/gmb.2025.10007.
Author contribution
Conceptualisation: Gisèle LaPointe. Methodology: Shadi Pakroo, Armin Tarrah, and Samira Soltani. Formal analysis: Armin Tarrah and Shadi Pakroo. Data curation: Gisèle LaPointe and Armin Tarrah. Writing – original draft: Shadi Pakroo, Samira Soltani, and Armin Tarrah. Writing – review and editing: Gisèle LaPointe and Armin Tarrah. Supervision: Gisèle LaPointe and Armin Tarrah. Funding acquisition: Gisèle LaPointe.
Funding
This study was funded by the NSERC Dairy Alliance (CRDPJ 529498-18) held by Gisèle LaPointe.
Data availability statement
The raw reads were deposited publicly in the Sequence Read Archive (SRA) database under the BioProject number “PRJNA1110247.”
Competing interests
The authors declare that there are no conflict of interests.
Ethical approval and consent to participate
All procedures were performed in compliance with relevant laws and institutional guidelines for the collection of human faecal samples, which were approved by the University of Guelph Research Ethics Board (Certificate No. 20-02-016) with informed consent obtained from the participant. The privacy rights of human subjects must be observed.








