Introduction
The gut microbiomes in most organisms are a rich source of bioactive compounds that have the potential to be developed into future antibiotics (O’Neill, Reference O’Neill2014). These compounds include antimicrobial peptides, bacteriocins, and other small bioactive molecules that can interact with other pathogenic bacteria to inhibit or modify their growth and colonization (Tortorella et al., Reference Tortorella, Tedesco, Espositom, January, Fani, Jaspars and de Pascale2018). There are billions of microbes present in the body that have coevolved with organisms and play a pivotal role in controlling many physiological activities, including the strengthening of the immune system. The emerging resistance to known antibiotics for preventing the recurrence of diseases in aquaculture farming and also for the need for new and green antibiotics has led to the discovery of new bioactive compounds from microbiome strains. The microbiome species produce several bioactive compounds in response to either pathogenic bacteria and viruses or other environmental stresses. All such bioactive compounds produced have significant functional properties like antimicrobials and antibiotics and can be used for therapeutic purposes to mitigate infectious diseases in cultivable organisms. In the early 1960 an antibiotic like penicillin was discovered by screening 19 microbiome species, and later several novel bioactive compounds and antimicrobial peptides were isolated from the microbiomes of soil and marine, and other diverse habitats (Moore and Gerwick, Reference Moore and Gerwick2012; Lam and Crawford, Reference Lam and Crawford2018). At present, several bioactive compounds are being discovered using a combination of genome mining, activation of silent biosynthetic pathways, and metagenomic analysis of a variety of ecosystems (Challinor and Bode, Reference Challinor and Bode2015; Garcia-Gutierrez et al., Reference Garcia-Gutierrez, Mayer, Cotter and Narbad2018). Even small biomolecules, peptides, and secondary metabolites are also being discovered from the microbiome species, and such compounds are obtained mostly from the commensal microbiomes and through microbe–host interactions. To understand the host-microbiome interaction and identify potential antimicrobial compounds focus of research is now given to large-scale genome sequencing of isolates of body parts of the cultivable organisms. Metagenomics and meta-transcriptome data collected from different commensal microbiomes were analyzed using bioinformatics applications to understand the functional aspects of molecules (Diwan et al., Reference Diwan, Harke and Panche2023, Reference Diwan, Harke and Panche2024).
In aquaculture farming, the surge of antimicrobial resistance infections and the concurrent increase in the usage of antibiotic drugs and other chemicals have jeopardized the healthcare system in cultivable organisms. This has created an unhealthy aquatic environment, creating opportunities for the entry of new pathogens. There is a need to build a robust healthcare system naturally, without using any drugs among the cultivable organisms, so that incidences of mass mortality and losses can be avoided. Therefore, the search for alternate antimicrobial agents has become a necessity. In recent years, emphasis has now been given to searching for natural products as sources of therapeutic agents, with antimicrobials being one of the most compelling biomolecules. Unlike microbial-originated antibiotics, plant-based antimicrobials have been extensively explored and with varied applications in medicine, veterinary, agriculture, and biotechnology. The microbiomes located in the gut system of animals have been recognized as producers of bioactive compounds with antibacterial, antifungal, and cytotoxic bioactivity (Xu et al., Reference Xu, Meng, Cao, Wang, Shan and Wang2015; El-Demerdash et al., Reference El-Demerdash, Tammam, Atanasov, Hooper, Al-Mourabit and Kijjoa2018; Karpinski, Reference Karpinski2019; Elissawy et al., Reference Elissawy, Dehkordi, Mehdi Nezhad, Ashour and Pour2021). Due to their distinctive biological properties, researchers have recently identified microbes as untapped reservoirs for novel antimicrobial agents (Swift et al., Reference Swift, Louie, Bowen, Olson, Purvine, Salamov, Mondo, Solomon, Wright and Northen2021). Specifically, the invention of state-of-the-art molecular biology, genetic, genomic, and computational tools has facilitated the mining of microbial structural systems to enhance drug discovery (Moir et al., Reference Moir, Shaw, Hare and Vovis1999; Schnappinger, Reference Schnappinger2015; Maghembe et al., Reference Maghembe, Damian, Makaranga, Nyandoro, Lyantagaye, Kusari and Hatti-Kaul2020).
The microbiome present in the gut system of fish and shellfish is ubiquitous, diverse community of organisms broadly categorized into viruses, bacteria, archaea, fungi, and protists. Among these microbiome communities, bacteria and fungi have been explored as potential sources of novel antimicrobial bioactive compounds. There are reports regarding the isolation of several peptide compounds (mathiapeptide, destotamide, Marfomycins, spirotetronates abyssomycin, and Lobophorin) from the bacteria including staphylococcus aureus, Micrococcus luteus, Bacillus subtilis, Enterococcus faecalis having the properties of antimicrobial compounds. Several researchers have conducted in vivo and in vitro studies to show that the bioactive compounds extracted from Cyanobacteria, yeast, and microalgae have antibacterial functions. Such discoveries of antimicrobial compounds from microbiome species have inspired several workers to produce synthetic antimicrobials from natural products to overcome antibiotic resistance (Mitcheltree et al., Reference Mitcheltree, Pisipati, Syroegin, Silvestre, Klepacki, Mason, Terwilliger, Testolin, Pote and Wu2021). Due to the emergence of diverse strains of microbiomes having antimicrobial properties and the advent of modern scientific tools for analysis, there is a large potential and opportunities to enhance the bioprospecting of new antimicrobial compounds. Therefore, the main objectives of this review are to investigate and report the novel antimicrobial compounds from the gut microbiome species of fish and shellfish of aquaculture importance and further explore their potential use for therapeutic purposes, replacing antibiotic drugs.
Antibacterial compounds from the bacterial community
Since the ban on antibiotics in aquaculture farming, emphasis is now being given to searching for natural products as sources of therapeutic agents to prevent the recurring spread of diseases in fish and shellfish during their captive culture. The plant-based anti-microbial has been extensively explored, and its therapeutic use in medicine, veterinary, agriculture, and biotechnology has been well-proved. However, anti-microbial compounds from microbiomes of fish and shellfish have not been explored much, and extensive research is warranted in this niche area of science (Diwan et al., Reference Diwan, Harke and Panche2023). The gut microbiome species present in fish and shellfish have been recognized as the producers of several novel bioactive compounds with antibacterial, antifungal, and cytotoxic functional properties (Xu et al., Reference Xu, Meng, Cao, Wang, Shan and Wang2015; El-Demerdash et al., Reference El-Demerdash, Tammam, Atanasov, Hooper, Al-Mourabit and Kijjoa2018; Karpinski, Reference Karpinski2019; Elissawy et al., Reference Elissawy, Dehkordi, Mehdi Nezhad, Ashour and Pour2021). They also produce functionally rich secondary metabolites, which enable them to survive in varied environmental conditions. Several reports in the recent past mention that microbes are unexplored reservoirs of novel antimicrobial agents due to their distinctive biological properties (Swift et al., Reference Swift, Louie, Bowen, Olson, Purvine, Salamov, Mondo, Solomon, Wright and Northen2021). Because of the advancement in analytical molecular tools, the innovative inventions in molecular genomics and genetics, and in-silico technology have facilitated the mining of microbial structural profiles to enhance drug discovery research (Moir et al., Reference Moir, Shaw, Hare and Vovis1999; Schnappinger, Reference Schnappinger2015; Maghembe et al., Reference Maghembe, Damian, Makaranga, Nyandoro, Lyantagaye, Kusari and Hatti-Kaul2020). The microbiome community in such cultivable aquatic organisms is ubiquitous, diverse in composition, and broadly categorized into viruses, bacteria, archaea, fungi, and protists. Predominantly, bacteria and fungi are explored as potential sources of novel antimicrobial agents. Sanchez et al. (Reference Sanchez, Wong, Romina, Christopher and Roger2012), while working on the marine fish gut microbiome, reported that several natural bioactive compounds produced by the gut microbiomes possess significant functional properties.
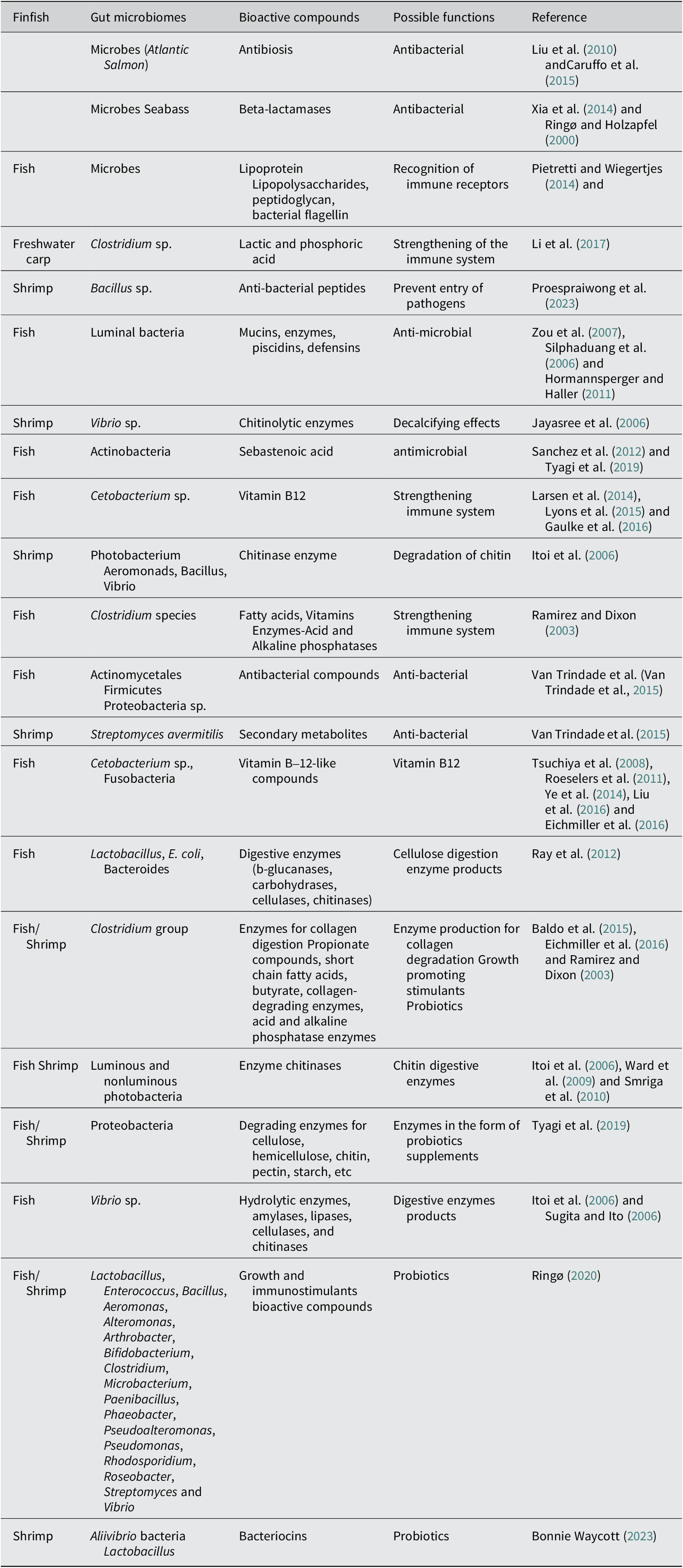
The microbiome belongs to the group Actinobacteria, including Rhodococcus, Microbacterium, and Micromonospora species, which are present in the digestive tract of six varieties of fish predominantly, and produces a novel lipid compound, viz., sebastenoic acid. Many researchers have reported that Sebastenoic acid has antibacterial properties that protect organisms against bacterial infection, particularly from strains like Staphylococcus aureus, Bacillus subtilis, Enterococcus faecium, and Vibrio mimicus. Diwan et al. (Reference Diwan, Harke, Gopalkrishna and Panche2021) in their review paper mentioned about 1000 new bioactive compounds produced by the microbiome species associated with several marine invertebrates. As the microbiome species produce several vital bioactive compounds having the properties of antimicrobials, a lot of research work has been done on genome sequencing of these microbiome communities (Van Trindade et al., Reference Van Trindade, Zyl, Navarro and Abd Elrazak2015). It has been further reported that 37 secondary metabolites have been discovered from the gene clusters of the fish gut microbiome species like Streptomyces avermitilis, having the properties of antimicrobials. The strains of firmicutes and proteobacteria from the gut of fish have been shown to contain active biological ingredients having functional properties against Gram-positive and Gram-negative pathogenic bacteria. Several peptides, alkaloids, and sesquiterpenes have been isolated from the different bacterial species, having the properties of antimicrobials (Tortorella et al., Reference Tortorella, Tedesco, Espositom, January, Fani, Jaspars and de Pascale2018). Furthermore, in vivo and in vitro assays have also demonstrated the anti-infective potential of other microbial products extracted from cyanobacteria, microalgae, and yeast (Rojas et al., Reference Rojas, Rivas, Cardenas and Guzman2020; Alsenani et al., Reference Alsenani, Tupally, Chua, Eltanahy, Alsufyani, Parekh and Schenk2020). Several workers have reported that the lactic acid bacteria found in the gut of fish produce bacteriocin compounds that are effective against pathogenic bacteria (Tenea et al., Reference Tenea and Yépez2016). Similarly, bacteriocin produced by Lactobacillus pentosus has been found to arrest the proliferation of certain pathogenic bacteria like E. coli, Pseudomonas aeruginosa, E. faecalis, K. pneumoniae, and Lactobacillus curvatus (Todorov and Dicks, Reference Todorov and Dicks2007). Bonnie Waycott (Reference Waycott2023) mentioned that the bacteriocin produced by the lactobacillus sp. can be used as an antibiotic alternative. It was confirmed by the application of bacteriocin in shrimp farming. Bacteriocins are low molecular weight polypeptides synthesized in ribosomes and contain 20–60 amino acid residues. Since the discovery of different forms of bacteriocin from microbial species and their antimicrobial properties, several workers have taken an interest in this product and worked out their mechanisms related to antimicrobials (Oscáriz and Pisabarro, Reference Oscáriz and Pisabarro2001; Cotter et al., Reference Cotter, Hill and Ross2005; Güllüce et al., Reference Güllüce, Karadayı and Barı2013; Herzog et al., Reference Herzog, Tiso, Blank and Winter2020; Jiang et al., Reference Jiang, Zu, Li, Meng and Long2020; Hernández-González et al., Reference Hernández-González, Martínez-Tapia, Lazcano-Hernández, García-Pérez and Castrejón-Jiménez2021; Thakur et al., Reference Thakur, Saini, Thakur, Gupta, Saini and Saini2021). These compounds have shown various biological activities against microorganisms, biofilm, tumors, and oxidation. Several biopharma industries are now looking for such new microbial products from the microbiome species of both fish and shellfish, and manufacturing these products under different commercial names for their use in aquaculture farming. To promote the prospects of such microbial products in these industries, there is an urgent need for intensive research on fish and shellfish microbiomes, particularly in genomics and metagenomics areas. The functional aspects of the gut microbiome are an important issue for future investigation, and therefore, in-depth knowledge of functional metagenomics is very much essential. Table 1 summarises fish and shellfish microbiomes producing bioactive compounds for their potential use in the development of aqua-bio industries. It has been reported that Actinobacteria found in the gut of several marine fish produce many bioactive compounds, including the most important one i.e., bacteriocin. Another compound called anthramycin which has the property of an antibiotic, is produced by actinomycete bacteria, which works against Bacillus anthracis, a pathogenic bacterium.
Table 1. Bioactive compounds derived from the gut microbiomes of finfish and shellfish
Anthramycin is also found to be produced by the gut bacterium Streptomyces from marine environments, particularly in higher organisms 65 (Valliappan et al., Reference Valliappan, Sun and Li2014). Actinobacteria from marine fish have been the focus of several researchers as this group of microbiomes produces many antibacterial compounds (Vignesh et al., Reference Vignesh, Ayswarya, Gopikrishnan and Radhakrishnan2019; Vadivel et al., Reference Vadivel, Venugopal, Angamuthu, Manikkam, Joseph and Aruni2021). The antimicrobial activity of Actinobacteria against pathogens like Salmonella enterica, S. aureus, E. coli, and Streptomyces bacterium, working as antimicrobial, antifungal, and quorum-sensing inhibitory activity, has been well-established in the recent past.
After realizing the importance of fish gut microbiomes as the best resources for producing novel bioactive compounds, one of the challenges researchers faced was how to culture these microbiome strains under lab conditions for their large-scale production and utilization in aquaculture to replace antibiotic drugs. It was also felt that the structure and functional properties of the bioactive compounds they produce in laboratory culture systems should not be altered as several environmental factors including the temperature, PH, and dissolved O2 concentration may affect not only the diversity of microbiome composition but also the nature of the bioactive compounds (Abe and Nakazawa, Reference Abe and Nakazawa1994; Lim et al., Reference Lim, Suh, Kim, Hyun, Kim and Lee1994; Knappe et al., Reference Knappe, Linne, Zirah, Rebuffat, Xie and Marahiel2008; Saalim et al., Reference Saalim, Villegas-Moreno and Clark2020). It is reported that in lab culture of such microbiome strains, often, modified or selective media are often used with long incubation times of days, or even weeks, for antimicrobial production to occur. Sanchez et al. (Reference Sanchez, Wong, Romina, Christopher and Roger2012), while working on the fish gut microbiome, used selective media for the lab culture of Actinobacteria to produce bioactive enzymes. For streptomyces culture in the lab, Vadivel et al. (Reference Vadivel, Venugopal, Angamuthu, Manikkam, Joseph and Aruni2021) mentioned that by substituting carbon, nitrogen, and salt sources as well as altering the pH, there was a drastic change in the antimicrobial properties of streptomyces. Uniacke-Lowe et al. (Reference Uniacke-Lowe, Stanton, Hill and Ross2024) while working on the gut microbiome of fish as a source of bacteriocins, reported that several environmental factors such as high salinity, hydrostatic pressure and a range of environmental temperatures all have an impact on the structural and functional diversity of marine antimicrobial molecules produced in the gut microbiomes, including bacteriocins. Further, it is mentioned that the characterization of the diversity of microbiome composition in such variable environments and the nature of the bioactive compounds they produce are still future challenges that require a lot of research investigations. In such challenges, advanced molecular tools like metagenomics, bioinformatics, and next-generation sequencing technologies will greatly support resolving the fish gut microbiome’s taxonomic diversity and identifying the microbiome ingredients with their functional aspects. Efforts have been made to summarise different antimicrobial compounds produced by fish and shellfish microbiomes in Figure 1.
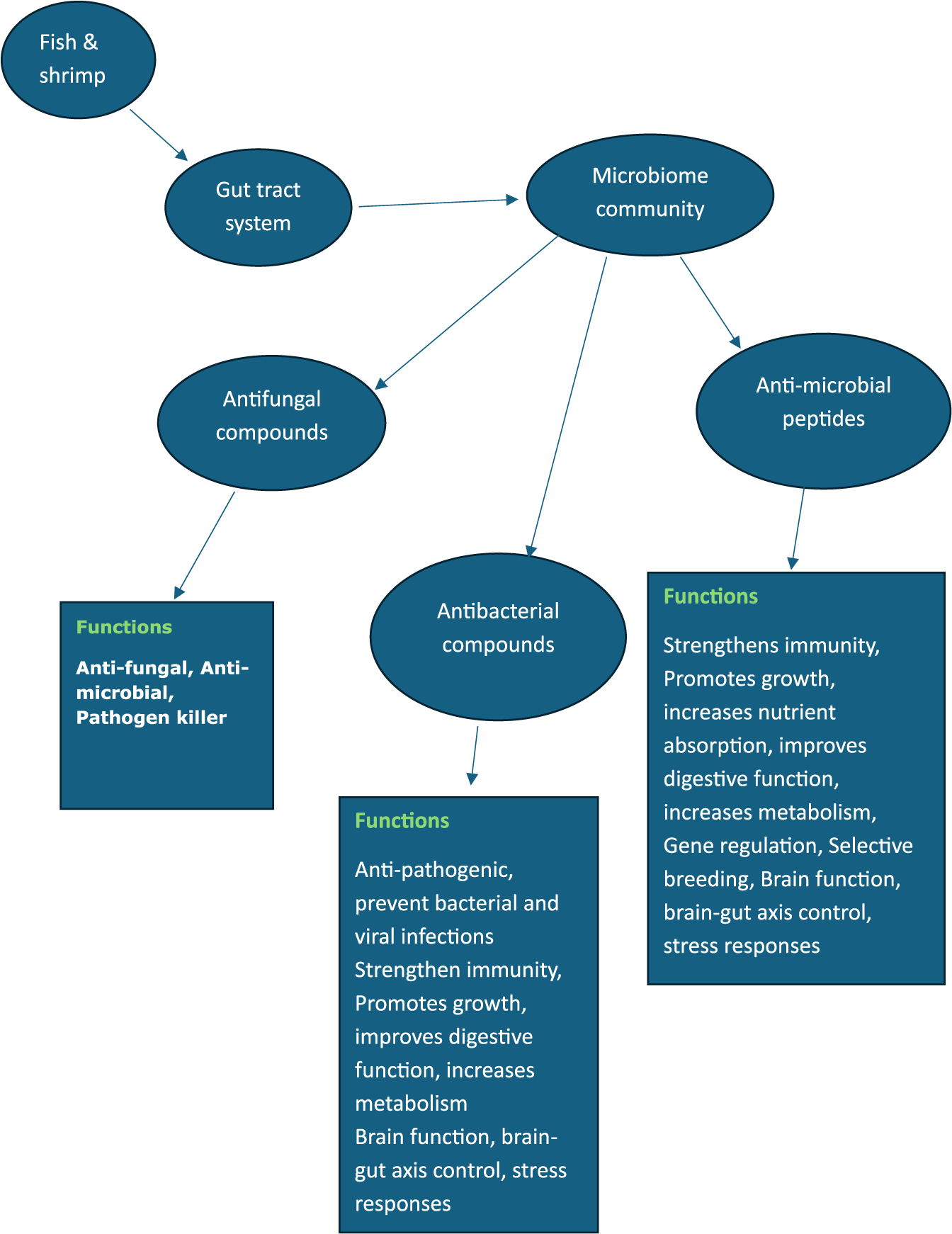
Figure 1. Illustrates the functional aspects of different microbial compounds produced by the gut microbiome community in fish and shrimp.
Antifungal compounds from the microbiomes
The fungal diseases in fish and shellfish are common and, if left untreated, can cause a secondary infection, leading to septicemia, fin rot, or dropsy. Further, it can severely damage the fish population and lead to heavy mortality in aquaculture farming systems. The fungus infection is a disease that affects mainly the skin and gills of fish and shellfish, and spores of the fungus cause fish fungus (Brown et al., Reference Brown, Denning, Gow, Levitz, Netea and White2012). Many different species of fungus can infect fish, and these include Saprolegnia, Achylia, and Fusarium. To prevent the spread of fungal diseases, standard protocols are available in the literature, including the use of antibiotics in severe cases of fungal infections. To replace antibiotics, research is being focused on using microbiome therapies or new antifungal drugs derived from the marine invertebrate (Zhang et al., Reference Zhang2020). There are reports that the killer fungus, Candida auris is spreading in healthcare facilities worldwide, and cautious threat alerts are being issued from time to time by the Centers for Disease Control and Prevention, USA (Meis and Voss, Reference Meis and Voss2019; Zhang et al., Reference Zhang2020) for controlling the spread of the fungus. However, preventing the spread of this fungus using multiple drugs has been tried by several researchers, but with limited benefits. In recent years because of the invention of molecular tools like liquid chromatography-mass spectrometry (LC–MS)–based metabolomics, and antimicrobial activity screening of metabolomic arrays from the microbiome isolates of marine animals (Hou et al., Reference Hou, Braun, Michel, Klassen, Adnani and Wyche2012; Chanana et al., Reference Chanana, Thomas, Braun, Hou, Wyche and Bugni2017), emphasis is now being given to finding out new natural bioactive compounds from the gut microbiome strains which are effective in preventing fungus infection.
Zhang et al. (Reference Zhang2020), while working on the discovery of a new anti-fungal drug from the marine microbiome, Micromonospora sp, found that turbinmicin is a promising antifungal bioactive compound. in vitro and in vivo, this compound exhibited powerful antifungal activity against emerging multidrug-resistant human fungal pathogens, including C. auris. Turbinmicin belongs to a small group of polyketides. It has been reported that this compound is highly antimicrobial and works effectively against C. auris, and Aspergillus fumigatus. This prominent functional antimicrobial property supports the compound’s development for future clinical use. Turbinmicin, when it was tested with different concentrations and doses on other fungal species like Candida albicans, C. glabrata, C. tropicalis, Aspergillus fumigatus, Fusarium spp., Scedosporium spp., and Rhizopus spp., was found to be very effective. Kingwell (Reference Kingwell2021), while working on the marine microbiome and antifungal properties, made a similar observation about the compound turbinmicin and its significant role in controlling the fungus infection caused by C. albicans. Another microbiome species from marine habitats Micromonospora, has been identified with the production of antifungal compounds. These species produce a secondary metabolite called spartanamycin, which is active against C. albicans, Aspergillus Cladosporium, and cryptococcus sp. (Kerr, Reference Kerr1999; Boumehira et al., Reference Boumehira, El-Enshasy, Hacene, Elsayed, Aziz and Park2016). Boumehira et al. (Reference Boumehira, El-Enshasy, Hacene, Elsayed, Aziz and Park2016) also reported that Micromonospora neiheumicin produces an antifungal compound, neiheumicin, which works against Saccharomyces cerevisiae. Some workers have reported that bacilli species are good sources of producing several antifungal compounds, like iturin, bacillomycin, mycosubtilin, and mojavensin (Kerr, Reference Kerr1999; Dunlap et al., Reference Dunlap, Bowman and Rooney2019). According to Kerr (Reference Kerr1999), anti-microbial compounds like azoxybacilin, bacereutin, cispentacin, and mycocerein are found to be produced by Bacillus cereus, and all these compounds work against Aspergillus species, Saccharomyces spp., Candida albicans, and other fungi. Chernin et al. (Reference Chernin, Brandis, Ismailov and Chet1996) reported that Enterobacter sp. produces a compound called herbicolins, which has been found to be active against yeasts and filamentous fungi. Similarly, Pseudomonas sp. has been reported to produce antimicrobial compounds, pseudomycin, caryoynencins, and cyclic hydroxamic acid (Vincent et al., Reference Vincent, Harrison, Brackin, Kovacevich, Mukerji, Weller and Pierson1991; Yamaguchi et al., Reference Yamaguchi, Park, Ishizuka, Omata and Hirama1995; Kerr, Reference Kerr1999).
Antimicrobial peptides from microbiomes
Several workers have reported that the microbiomes produce various antimicrobial peptides and play a significant role in the host defense systems (Wang et al., Reference Wang, Dou, Song, Lyu, Zhu, Xu, Li and Shan2019). These antimicrobial peptides are small-sized protein compounds consisting of large numbers of lysine and arginine residues, which are cationic. These compounds positively charged cationic properties enable them to react with microbial membranes of infectious bacteria that are negatively charged (Narayana and Chen, Reference Narayana and Chen2015). In aquaculture farming, infection with bacteria and viruses is so common, and every time, there is no alternative technology except to use antibiotic drugs to control such infectious diseases. However, after discovering antimicrobial peptides from microbiome species and their vital role in controlling diseases, much attention is now paid to replacing antibiotic drugs with antimicrobial peptides (Danquah et al., Reference Danquah, Minkah, Osei Duah Junior, Amankwah and Somuah2022). Recently, antimicrobial peptides have shown excellent antibacterial activity against harmful pathogenic microorganisms by acting on multiple target points (Huerta-Cantillo and Navarro-García, Reference Huerta-Cantillo and Navarro-García2016; da Cunha et al., Reference da Cunha, Cobacho, Viana, Lima, Sampaio, Dohms, Ferreira, de la Fuente-Núñez, Costa and Franco2017). A few reports also indicated that antimicrobial peptides act as antifungal, antiviral, antiparasitic, and immunomodulatory agents (Wang et al., Reference Wang, Dou, Song, Lyu, Zhu, Xu, Li and Shan2019). Even common pathogenic bacteria like Acinetobacter baumannii, Listeria monocytogenes, E. coli, and Vibrio parahaemolyticus are affected and controlled by antimicrobial peptides. The antimicrobial peptides like nisin, cecropins, and defensins have shown excellent results in preventing infection caused by Gram-positive and Gram-negative bacteria. 104 Though applications of antimicrobial peptides are diverse, their potential use has good scope not only in the aquaculture industry but also in human medicines, the food industry, agriculture sector; however, their production at the industrial scale is low. One reason speculated for low production is that the antimicrobial peptides are susceptible to proteolytic degradation due to the L-amino acids in them (da Cunha et al., Reference da Cunha, Cobacho, Viana, Lima, Sampaio, Dohms, Ferreira, de la Fuente-Núñez, Costa and Franco2017). Hence, genetic engineering tools are now being used to increase the production of antimicrobial peptides with better functional properties.
Therapeutic applications
It is well-established that the commensal microbiome community available in the gut tract of fish and shellfish plays a significant role in building strong immunity to manage the prevention of infectious diseases and keep the animal’s body healthy. Therefore, in aquaculture farming, several researchers have focused on investigating the possible role of antimicrobial compounds produced by the microbiome species in fish gut tracts in controlling bacterial and viral diseases. Many investigations in the recent past have also emphasized their research programs on interactive mechanisms between commensal microbiomes and pathogenic bacteria and viruses (Diwan et al., Reference Diwan, Harke and Panche2023). Zhang et al. (Reference Zhang, Salinas and Li2010) and Salinas et al. (Reference Salinas, Zhang and Sunyer2011) discovered a compound called “immunoglobulin” while working on fish microbiomes, and this compound they derived from the interaction between commensal and pathogenic bacteria present in mucosal layers of the skin and other tissues. Further, these authors have mentioned whether the mechanism involved in immunoglobulin production by the microbes present in the gut tract and skin tissues in response to pathogenic bacteria is similar to the mechanisms that also exist for the gill tissues. Zhang et al. (Reference Zhang, Salinas and Li2010) reported the presence of different types of immunoglobulins in the gill tissues of rainbow trout, performing the function of defense, and from these findings, it was speculated that the possibility of developing a cost-effective vaccine from microbiomes for controlling infectious diseases (Rawls et al., Reference Rawls, Samuel and Gordon J2004). Many findings suggest that the genes in the fish body that control different physiological activities in the digestive tract, including functions like immunity, nutrition, and metabolism, are also governed by the gut microbiomes. Hence, there is an urgent need to identify these microbiomes, their ingredient molecules, functions, and their interaction with the host microbiome. Perez et al. (Reference Perez, Balcazar, Ruiz-Zarzuela, Halaihel, Vendrell and Iú de Blas2010) found that the lymphoid tissues that are associated with the gastrointestinal tract can identify the pathogenic bacteria in the gut system, and at the same time, the commensal microbiomes regulate the immune system in fish. From some of these observations, it was inferred that the microbiome present in the intestinal mucosal layers produces many antimicrobial compounds mucins, enzymes piscidins, and defensins in response to pathogenic bacteria, and this sort of reaction is the first line of defense provided in fish and the second line of defense is the lymphoid tissues which produce many immunoglobulins as mentioned earlier (Silphaduang et al., Reference Silphaduang, Colorni and Noga2006; Zou et al., Reference Zou, Mercier, Koussounadis and Secombes2007). It has been observed that the continuous use of antibiotic drugs, vaccines, and other chemotherapeutic methods to control the frequent occurrence of bacterial and viral diseases in aquaculture farming has offered limited solutions and allowed the entry of more infectious new pathogens. Therefore, new methods and protocols based on natural compounds are necessary for the purpose. Becattini et al. (Reference Becattini, Taur and Pamer2016) while working on fish microbiomes reported that the microbe, Clostridium butyricum produces short-chain fatty acids that help the animal not only provide energy for the regeneration and repair of epithelial cells of the gut tract but also to reduce the pH of intestinal fluid, promoting the growth of bacteria, and preventing the entry of invasive pathogens. There are also reports that C. butyricum produces bacteriocin and lipoteichoic acid that act as antibacterial compounds (Gao et al., Reference Gao, Wu and Wang2011, Reference Gao, Xiao, Sun, Peng, Yin and Ma2013; Junghare et al., Reference Junghare, Subudhi and Lal2012). Applications of C. butyricum in preventing Vibrio harveyi attack in freshwater prawn Macrobrachium rosenbergii have been reported by Sumon et al. (Reference Sumon, Ahmmed, Khushi, Ahmmed, Rouf and Chisty2018). Duan et al. (Reference Duan, Dong, Wang, Li, Liu and Zhang2017) also observed that the presence of C. butyricum in the shrimp is advantageous in preventing bacterial and viral infection, strengthening the immune system, and managing thermal stress. The production of antibacterial compounds like crustin, prophenoloxidase enzymes, lysozymes, and glucan-binding proteins from C. butyricum in the gut tract of shrimp and their role in controlling infectious diseases have been reported by Miandare et al. (Reference Miandare, Mirghaed, Hosseini, Mazloumi, Zargar and Nazari2017) and Chen et al. (Reference Chen, Chen, Kuo, Lin, Chang and Gong2016). While investing in the health benefits of the bacterium C. butyricum and the butyrate in fish and shellfish, Tran et al. (Reference Tran, Huifen, Jinkun, Taoqiu, Zhang and Shengkang2023) in their paper mentioned that this bacterium can enhance the growth performance of cultured animals. This is because C. butyricum facilitates the structural modification of surface layers in the intestine and the activity of digestive enzymes, as well as modulating gut microbiota, with an increase in the commensal bacteria and a decrease in the population of infectious pathogens. Having realized the importance of C. butyricum, it is necessary to prioritize further research on this bacterium’s genomic and metagenomics profile so that such studies will help us in the larger production of antibacterial compounds for their wide application in controlling diseases. Similar research work must be planned to investigate more gut microbiome species that are involved in antibacterial functions.
Though a considerable amount of information is now available covering several aspects of the gut microbiomes in fish and shellfish, research on the functional aspects of microbial species at the genomic level is lacking. Several reports indicated that the gut microbiome plays an important role in maintaining the proper growth and health of the animals. In earlier years, they indicated that the gut microbiome in fish and shellfish produces various digestive enzymes. However, some enzymes like β-glucanases, carbohydrases, cellulases, and chitinases fish do not produce particularly herbivorous and detritivorous species. In such animals, the digestion of cellulose is taken over by the microbiome species present in the gut system at that time (Ray et al., Reference Ray, Ghosh and Ringø2012). Tsuchiya et al. (Reference Tsuchiya, Sakata and Sugita2008) reported that microbiome species like Cetobacterium somerae present in the gut tract produce an abundant amount of vitamin B12. Findings of Cetobacterium producing vitamin B12 have been supported by Romero et al. (Reference Romero, Ringø, Merrifield, Merrifield and Ringø2014), who also mentioned that this bacterium produces vitamin B12 in fish like Nile tilapia and common carp Cyprinus carpio, which have no dietary vitamin B12 requirement. However, fish species like channel catfish Ictalurus punctatus, and Japanese eel Anguilla japonica, in which Cetobacterium is not commonly present in their gut system, require dietary vitamin B12 from external sources. Some other workers reported that the production of various short-chain fatty acids occurs when gut tract microbes are involved in the digestion of dietary fiber, particularly in herbivorous fish species (Mountfort et al., Reference Mountfort, Campbell and Clements2002).
Several reports have described the functional aspects of short-chain fatty acids in many fish, emphasizing that the presence of short-chain fatty acids can make the environment non-conducive to some potential pathogens and increase the solubility of minerals, making them more easily absorbed (Mountfort et al., Reference Mountfort, Campbell and Clements2002; Merrifield and Rodiles, Reference Merrifield, Rodiles, Benjamin and Peatman2015). Certain findings have confirmed that the fishes cannot digest food containing cellulose and degrade and eliminate xenobiotic compounds from their body without certain gut microbiome species. Few reports mention the digestion of cellulose materials and converting them into β-glucans and β-glucose in the presence of Verrucomicrobia bacteria in the gut of the fish. These findings confirmed that they are important for digesting plant cellulose in the fish gut. These aspects have been experimentally proved in carp and further strengthened by reduced cellulase activity in antibiotic-treated fish (van Kessel et al., Reference Van Kessel, Dutilh, Neveling, Kwint, Veltman, Flik, Jetten, Klaren and Op den Camp2011). Eichmiller et al. (Reference Eichmiller, Hamilton, Staley, Sadowsky and Sorensen2016) noted that microbes belonging to the Clostridia group produce several bioactive compounds, including propionate, short fatty acid chains, and butyrate, within the host gut tract system to maintain and promote the host’s growth. Identical observations have been made by Baldo et al. (Reference Baldo, Riera, Tooming, Alba and Salzburger2015) while working on these bacteria, which perform the function of collagen digestion as they produce collagen-degrading enzymes. Several workers have reported that the Fusobacteria present in the gut tract of fish produce vitamin B12, which is vital in performing the function of the growth and development process (Roeselers et al., Reference Roeselers, Mittge, Stephens, Parichy and Cavanaugh2011; Ye et al., Reference Ye, Amberg, Chapman, Gaikowski and Liu2014; Eichmiller et al., Reference Eichmiller, Hamilton, Staley, Sadowsky and Sorensen2016; Liu et al., Reference Liu, Guo, Gooneratne, Lai, Zeng, Zhan and Wang2016).
Advanced tools for the analysis of antimicrobial compounds
The antimicrobial compounds from microbiomes and their functional role in antimicrobial resistance are well understood, however, their large-scale applications in aquaculture farming and other sectors have been hindered due to less investment and the high cost of production of such compounds from natural resources. In addition to this, we also need innovative and sophisticated advanced techniques for their discovery and analysis, particularly from the gut microbiome species of fish and shellfish. Traditional methods of analysis of antimicrobial compounds from microorganisms include several types of diffusion methods. These methods are agar disk diffusion, agar well diffusion, antimicrobial gradient, and agar plug diffusion (Rex et al., Reference Rex, Ghannoum, Alexander, Andes, Brown, Diekema, Espinel-Ingroff, Fowler, Johnson and Knapp2010; Balouiri et al., Reference Balouiri, Sadiki and Ibnsouda2016). The agar disk diffusion method is the most commonly used test to assess pathogen susceptibility, and in this method, a desired concentration of the compound to be tested is placed on the surface of agar containing microbes. Here, antimicrobial agents enter the test compound, diffuse into the agar, and inhibit the proliferation of susceptible microbes, which can be measured. Though his method cannot accurately determine the minimal inhibitory concentration, it is simple and less expensive (Weinstein et al., Reference Weinstein, Patel, Bobenchik, Campeau, Cullen, Gallas, Gold, Humphries, Kirn and Lewis2019). Another method is the antimicrobial gradient, which combines dilution and diffusion to determine the inhibitory concentration value of antibiotics and antimicrobial activity, including antifungal. The microbe’s cell damage and viability and its antimicrobial resistance capacity can be determined by the flow cytofluorometric protocol (Weinstein et al., Reference Weinstein, Patel, Bobenchik, Campeau, Cullen, Gallas, Gold, Humphries, Kirn and Lewis2019; Landaburu et al., Reference Landaburu, Berenstein, Videla, Maru, Shanmugam, Chernomoretz and Agüero2020). Therefore, in summary, traditional protocols for determining antimicrobial resistance of the microbes have been replaced in the recent past by more advanced tools like bioinformatics-based subtractive genomics and metabolic pathway analyses. (Uddin et al., Reference Uddin, Saeed, Khan, Azam and Wadood2015; Zhan et al., Reference Zhan, Pannecouque, De Clercq and Liu2016; Khan et al., Reference Khan, Mahmud, Iqbal, Hoque and Hasan2020; Shahid et al., Reference Shahid, Shehroz, Zaheer and Ali2020). However, the use of protocols based on in silico approaches is more advantageous in the future, but the full knowledge of their capabilities has not been explored yet. Nonetheless, other molecular and genomic technologies have recently seen some success.
Several workers have mentioned earlier that infection-based antimicrobial activity has enhanced the development of identification of promising therapeutics protocols, in the management of microbial diseases (Hackbarth et al., Reference Hackbarth, Chen, Lewis, Clark, Mangold, Cramer, Margolis, Wang, Koehn and Wu2002), as well as in the investigation of antibacterial inhibitors like peptide deformylase, which is a vital ingredient in the survival of pathogenic strains, such as Mycobacterium smegmatis (Chen et al., Reference Chen, Hackbarth, Ni, Wu, Wang, Jain, He, Bracken, Weidmann and Patel2004; Teo et al., Reference Teo, Thayalan, Beer, Yap, Nanjundappa, Ngew, Duraiswamy, Liung, Dartois and Schreiber2006; Kaplan et al., Reference Kaplan, Albert, Awrey, Bardouniotis, Berman, Clarke, Dorsey, Hafkin, Ramnauth and Romanov2012; Naor et al., Reference Naor, Gadot, Meir and Barkan2019). In recent years, Fanelli et al. (Reference Fanelli, Pappalardo, Chine, Gismondi, Neglia, Argentiero, Calderaro, Prati and Esposito2020) mentioned that due to the emergence of molecular tools, notably genomics, transcriptomics, and proteomics, has gained momentum in the development of bioinformatics knowledge to identify novel drugs and several other lead bioactive compounds that have the properties of antimicrobials. Further, it is also mentioned that genome mining technologies can be used to detect and analyze the biosynthetic gene clusters of such antimicrobial compounds. Vamathevan et al. (Reference Vamathevan, Clark, Czodrowski, Dunham, Ferran, Lee, Li, Madabhushi, Shah and Spitzer2019) emphasized that artificial intelligence and machine learning technologies also allow scientists to develop alternate protocols to battle against antimicrobial resistance using microbiome ingredients.
Challenges and future prospects
Several efforts are being made to replace antibiotic drugs and chemicals with natural products without diluting the impact of preventing bacterial and viral infectious diseases in fish and shellfish for the development of sustainable aquaculture all over the world. Many have suggested the use of plant-based phytochemical compounds, and there are reports on the use of marine bioactive compounds derived from various sources of animals and microorganisms. However, all these products derived from natural resources are still experimental stage and may take a long time to become a reality. Research investigations on the use of bioactive compounds derived from gut microbiomes of fish and shellfish for preventing infectious diseases in aquaculture farming have gained momentum in the recent past and several products like probiotics, prebiotics, synbiotics, bacteriophage technology, etc., are now available in the market and are being used in the aquaculture farming. However, using these products has its limitations, with variable results on the growth and performance of cultured organisms. Some antimicrobial compounds like bacteriocins, sebastenoic acid, spartanamycin, anthramycin, and turbinmicin, which have been discovered in microbiomes and experimentally proved to have great potential to replace antibiotic drugs, need further research investigations. The genome mapping, transcriptome studies, and metagenomics studies of such antimicrobial-producing microbiome species are essential and to be done on a priority basis. The number of novel bioactive compounds mentioned in this review illustrates the importance of the antibiotic properties of the gut microbiome species and some innovative ways the search for new antimicrobials in the management of various infectious diseases. Further investigation of these microbiomes will pave the way for searching for new antimicrobial compounds and present us with potent antimicrobials. With such novel compounds, antimicrobial resistance can be reduced among cultivable species in aquaculture farming. Investigations of such antimicrobial bioactive compounds from the microbiome communities will unlock a new innovative concept in animal health management and aquaculture farming, with effects on cost efficiency, species health, productivity, and yield enhancement. Therefore, introducing such new methods for therapeutic purposes in the treatment of bacterial diseases in cultivable organisms will be highly beneficial to the aquaculture industry.
Another challenge in investigating antimicrobial compounds from microbial communities in the marine environment includes the mass culture of the microbes and the expression of genes responsible for producing antimicrobial compounds under in vitro conditions. Because under variable environmental conditions, there is a possibility of changing the structural profile of microbiomes and their metabolites. Several ecological factors significantly disturb microbial diversity and the bioactive compounds/metabolites they produce. It has been suggested that the use of some selective and targeted media for the isolation of bioactive marine Actinobacteria from fish needs further investigation. It has also been observed that the presence of specific signaling molecules found in the bacteria inhabiting their natural environment has a direct impact on the growth of some marine bacterial flora. Advanced molecular technologies, such as next-generation sequencing, metagenomics, metabolomics, and genome mining, are more frequently being used to assess in-depth knowledge of microbial communities. In the recent past, many studies have emphasized the use of metagenomic sequencing and bioinformatic tools to characterize the functionality of microbial communities, antimicrobial resistance genes, and bioactive metabolite genes in marine organisms, including those from rarer deep-sea fish species.
Acknowledgements
All the authors sincerely acknowledge their gratitude and thanks to Shri Ankushrao Kadam, Chancellor and Secretary, MGM University, Aurangabad, Maharashtra, India, for his cooperation, constant encouragement, and for providing all the facilities during manuscript preparation.
Author contribution
A. D. Diwan is the main author who has articulated the concept of the theme of this paper, and he wrote and drafted the manuscript. S. N. Harke has helped collect the literature, supervised the work, and validated the manuscript. Archana Panche has equally contributed to the preparation of the manuscript.
Funding
For writing this review there was no funding from any agency.
Ethics approval
As this is a review paper, a declaration of ethics is not applicable.




