Management Implications
The development of advanced management interventions for dealing with invasive plants requires a more holistic approach that recognizes the multifaceted effects that such invasion processes might have, including potentially negative, neutral, or positive impacts, both above- and below-ground. To date, however, such evidence is scarce and often focused solely on above-ground effects and changes in the resident plant community with little information on soil organisms or interactions between different trophic levels that are involved in decomposition processes. This is particularly important, because a common feature of many plant introductions is a dramatically enhanced and often qualitatively modified litter production whose decomposition will likely have knock-on effects on soils and soil organisms, as well as on the drivers of soil biogeochemical processes. In this study, we show that Gunnera tinctoria (Chilean rhubarb) invasions can result in a shift in the soil biota and modifications in the detrital food chain, with an increased role for earthworms in decomposition processes and changes in the soil microbial population. This was linked to a 20-fold higher and qualitatively superior (i.e., lower carbon:nitrogen [C:N] ratio) litter production compared with that found in uninvaded grasslands. These largely below-ground effects, driven mainly by the increased inputs of higher-quality litter, may compromise the effectiveness of any measures aimed at restoring the original plant community. Thus, the removal of plants might lead to C loss and a reduction in earthworm and enchytraeid populations. Our results extend the understanding of the complexity of impacts that an invasive species may have on ecosystems and their processes, highlighting the need for more work that encompasses a wide range of invasive species to assess the generality of these results and that tackles multiple aspects of invasion processes at the same time.
Introduction
Invasive plants are known to have a range of impacts on ecosystems and the services they provide, although much of the focus has been on their effects on the standing vegetation and resident animals (Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011), while much less is known about below-ground effects (McCary et al. Reference McCary, Mores, Farfan and Wise2016). Interestingly, the effects on the above-ground vegetation/animals are overwhelmingly negative (Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011), while the impacts below-ground appear to be small, insignificant, or variable (McCary et al. Reference McCary, Mores, Farfan and Wise2016; Pearson et al. Reference Pearson, Minor, Robertson and Clavijo-McCormick2024). Although documented changes in soil chemical properties (Ehrenfeld Reference Ehrenfeld2003, Reference Ehrenfeld2010) and shifts in plant communities and their composition (Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011) might impact on the soil fauna (Zhang et al. Reference Zhang, Li, Wu and Hu2019) and soil bacterial communities (Gibbons et al. Reference Gibbons, Lekberg, Mummey, Sangwan, Ramsey and Gilbert2017; McTee et al. Reference McTee, Lekberg, Mummey, Rummel and Ramsey2017), few studies have specifically focused on below-ground impacts and their effects on distinct trophic levels at the same time.
Of particular importance, as far as energy flow and nutrient cycling are concerned, is the detrital (decomposer) food chain. This is a predominantly soil/below-ground process that is involved in the recycling of nutrients and represents the major energy flow within a terrestrial ecosystem. It is driven largely by litter inputs and the activity of soil organisms, and because plant invasions are typically associated with high litter inputs (Wolkovich et al. Reference Wolkovich, Lipson, Virginia, Cottingham and Bolger2010), this pathway may be of even greater significance for plant invaders. The extent to which invasive plants lead to ecosystem impacts via nutrient enrichment and associated modifications in soil biogeochemistry and its biota may depend, therefore, on the extent of decomposition of the generally larger litter inputs. Around 90% of nutrient cycling is thought to be associated with decomposition processes (Mary et al. Reference Mary, Recous, Darwis and Robin1996). For many highly productive plant invaders that have few natural enemies, such as Chilean rhubarb [Gunnera tinctoria Molina (Mirb.), Gunneraceae], grazing losses are likely to be less significant, placing an even greater emphasis on the detrital food chain.
Although the focus of many decomposition studies has been on the role of microorganisms, such as bacteria and fungi, the soil fauna can also play an important role (Griffiths et al. Reference Griffiths, Ashton, Parr and Eggleton2021). There is an increased recognition, for instance, of the role of earthworms in primary decomposition processes (Curry and Schmidt Reference Curry and Schmidt2007). Recent evidence also suggests that earthworms can rapidly transfer nutrients to plants and are not solely restricted to slow decomposition processes (Shutenko et al. Reference Shutenko, Andriuzzi, Dyckmans, Luo, Wilkinson and Schmidt2022). Importantly, there is also evidence that earthworms can drive the composition of soil bacterial communities through their contribution to the early stages of organic matter degradation (Medina-Sauza et al. Reference Medina-Sauza, Álvarez-Jiménez, Delhal, Reverchon, Blouin, Guerrero-Analco, Cerdán, Guevara, Villain and Barois2019). Their much smaller relatives, enchytraeid or pot worms (family Enchytraeidae), are studied less often but are likely similarly important (Didden Reference Didden1993); they were therefore also included in the present study.
Gunnera tinctoria is a South American species that has become invasive in Ireland, the United Kingdom, France, Portugal (Azores Archipelago), New Zealand, and the United States (Gioria and Osborne Reference Gioria and Osborne2013). This species forms monospecific stands and invades a wide range of habitats, including cliffs, ditches, and stream banks in environments typically characterized by high rainfall and moderate temperatures (Gioria and Osborne Reference Gioria and Osborne2013), impacting particularly on grasslands in Ireland due to its huge biomass productivity (Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020). Gunnera tinctoria belongs to the family Gunneraceae, the only angiosperm genus known to establish an endosymbiotic relationship with the cyanobacterium Nostoc punctiforme (Osborne and Bergman Reference Osborne and Bergman2009; Santi et al. Reference Santi, Bogusz and Franche2013), which gives it the ability to obtain nitrogen (N) from the atmosphere via N-fixation (Osborne et al. Reference Osborne, Doris, Cullen, McDonald, Campbell and Steer1991; Osborne and Sprent Reference Osborne and Sprent2002), and may be partially responsible for its success as a plant invader (Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020). This invasive plant species excludes native plants due largely to the development of an extensive canopy that shades out co-occurring species (Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020), alters the local soil greenhouse gas balance (Mantoani and Osborne Reference Mantoani and Osborne2021a), potentially enhancing N2O emissions through facilitated mechanisms (Mantoani and Osborne Reference Mantoani and Osborne2021b), and survives extreme weather conditions, enabling it to recolonize areas quickly (Mantoani and Osborne Reference Mantoani and Osborne2022; Mantoani et al. Reference Mantoani, Sweeney and Osborne2025).
In a recent paper, we showed that invasions by G. tinctoria can result in a marked increase in the abundance and diversity of native earthworms, alongside a 20-fold annual increase in plant litter (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022). However, earthworms can also feed on roots, seeds, fungi, and soil bacteria (Curry and Schmidt Reference Curry and Schmidt2007). To confirm and expand these results, we now provide evidence using stable isotope ratio measurements. For this, we tested the following hypotheses: (1) earthworms and enchytraeids feed directly on the G. tinctoria litter, which can be detected by stable isotope ratio measurements; (2) earthworm populations have the capacity to consume most of the litter produced by the plant invader (Curry and Schmidt Reference Curry and Schmidt2007); and (3) earthworm-and enchytraeid-related changes in the detrital food chain have knock-on effects on the soil microbial communities, as well as on N cycling (Jang et al. Reference Jang, Xiong, Liu, Yoo and Ishii2022).
Material and Methods
Area of Study and Experimental Design
The study area is the same as that reported in Mantoani et al. (Reference Mantoani, González, Sancho and Osborne2020, Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022, Reference Mantoani, Sweeney and Osborne2025) and Mantoani and Osborne (Reference Mantoani and Osborne2021a, Reference Mantoani and Osborne2022) and is located in Dooega Beach, Achill Island (53.51°N and 54.01°N, 9.955°W and 10.15°W), County Mayo, on the west coast of Ireland. Five replicate fields containing two plots of 5 by 5 m each (25 m2), one in uninvaded semi-natural grasslands (GRASS) and another one in areas invaded by G. tinctoria (GUN) were used in the study (n = 5 for each treatment). The distance between treatment plots was ca. 10 m, and replicates were at least 100 m apart from each other. Invaded plots were characterized by having a high coverage of G. tinctoria (i.e., 100% in summer) and low regeneration of native species (ca. 10 species or fewer), only being colonized by common bentgrass (Agrostis capillaris L.), creeping bentgrass (A. stolonifera L.), common horsetail (Equisetum arvense L.), water horsetail (E. fluviatile L.), dwarf willowherb (Epilobium obscurum Schreb.), cleavers (Galium aparine L.), common rush (Juncus effusus L.), meadow buttercup (Ranunculus acris L.), creeping buttercup (R. repens L.), and nettle (Urtica dioica L.), whereas uninvaded ones had 27 to 32 species that were absent in invasive stands (Gioria et al. Reference Gioria, Carta, Balogianni, Fornara, Pyšek and Osborne2023; Mantoani and Osborne Reference Mantoani and Osborne2022; Mantoani et al. Reference Mantoani, Sweeney and Osborne2025). In addition to the species present in invaded plots, as mentioned above, uninvaded grasslands were characterized by native grasses such as meadow foxtail (Alopecurus pratensis L.), sheep’s-fescue (Festuca ovina L.), Yorkshire fog (Holcus spp.), perennial ryegrass (Lolium perenne L.), meadow-grass (Poa spp.), as well as common Irish grassland species, for example, wild angelica (Angelica sylvestris L.), fool’s-water-cress (Apium nodiflorum (L.) Lag.), marsh thistle [Cirsium palustre (L.) Scop.], spear thistle [C. vulgare (Savi) Ten.], purple loosestrife (Lythrum salicaria L.), water mint (Mentha aquatica L.), common self-heal (Prunella vulgaris L.), bramble (Rubus fruticosus L.), common sorrel (Rumex spp.), red clover (Trifolium pratense L.), and white clover (T. repens L.). Local annual rainfall is above 1,100 mm, and the temperature ranges from 3 to 6 C in winter and from 12 to 15 C in summer (Gioria and Osborne Reference Gioria and Osborne2013). On Achill Island, G. tinctoria invades alluvial or colluvial soils, derived from thin gley material of marine origin or from volcanic material, with a pH between 4.6 to 6.2 (Gioria and Osborne Reference Gioria and Osborne2013).
Earthworm Sampling, Plant Harvesting, and Isotope Ratio Analyses
Following the sampling protocol described in Mantoani et al. (Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022), earthworms (family Lumbricidae) and enchytraeid worms (family Enchytraeidae) (both subclass Oligochaeta) were collected in June 2017 for isotopic analyses. In brief, soil blocks (25 by 25 by 30 cm) were hand sorted, and adults of Allolobophora chlorotica (a small, endogeic, soil-feeding species) and of Lumbricus rubellus (a medium-sized, epigeic, litter-feeding species), as well as enchytraeid worms that were visible under field conditions were collected manually. Both earthworm species are native to Ireland; enchytraeid species were not determined. All worms were kept live in a cooler box, transported to the laboratory, and allowed to void their guts on moist paper tissue for 24 h. Live plant material was also collected from both treatments and separated into leaf, petiole, and rhizome for G. tinctoria and leaves and roots for grassland plants.
Soil samples and rinsed plant materials were oven-dried (50 C) and powdered using a ball mill, while earthworms were freeze-dried and crushed individually using a mortar and pestle. Subsamples of about 1 mg dry weight (earthworms) and 4 mg dry weight (soil and plants) were weighed into miniature tin capsules (4 by 6 mm) and sealed. Several freeze-dried enchytraeid worms were pooled to achieve about 1 mg dry weight per cup. Samples were analyzed with elemental analysis–continuous flow isotope ratio mass spectrometry (EA-CF-IRMS) in dual isotope mode (Europa Scientific EA and Europa Scientific 20-20 IRMS, Sercon Ltd., Crewe, United Kingdom and the sample analyses carried out by Iso-Analytical Ltd., Cheshire, United Kingdom). Isotope ratios (13C/12C and 15N/14N) are expressed in conventional delta (δ) notation in parts per mille (‰). Analytical precision (SD, n = 8) of check samples (wheat [Triticum aestivum L.] flour) analyzed along with the samples was 0.01‰ and 0.08‰ for δ13C and δ15N, respectively. More details on the isotopic analysis are reported in Mantoani et al. (Reference Mantoani, González, Sancho and Osborne2020).
Soil Sampling: Soil Profile and Bulk Density
Soil samples were taken from three soil depths: 5 to 10, 20 to 25, and 50 to 55 cm. Samples were collected at each of the four corners of the plots and in their centers (see Kiely et al. Reference Kiely, McGoff, Eaton, Xu, Leahy and Carton2009), giving a total of 15 samples for chemical analyses and another 15 samples for the determination of soil bulk density in each of the plots. To verify whether there was any seasonal difference between winter and summer, we collected samples in January and July of 2016, with a total of 300 soil samples for each season and a total of 600 soil samples for the whole experiment. To reduce the effects of spatial variability within the plots, the data from each of the four corners and the centers of the plots were averaged (see Wellock et al. Reference Wellock, Rafique, Peichl and Kiely2014). A hammer auger coupled with a metal ring of known volume (i.e., 88.62 cm3) was used to collect samples for soil bulk density, while a Dutch auger was used to collect samples for the chemical analyses. Soil bulk density was determined by dividing the final weight by the initial volume after samples were dried until constant weight in a forced-draft oven at 70 C.
Soil Chemical Analyses: Carbon and Nitrogen
Soil carbon (C) and N determinations were made on soil samples that were first sieved (2-mm aperture) to remove stones and plant material, and then air-dried before grinding them into a fine powder using a soil mill. Soil C and N were determined using a CHN elemental analyzer (LECO, TruSpec, Nether Alderley, United Kingdom) to estimate total carbon (TC) and total nitrogen (TN). Fresh soil samples were also extracted using 0.5 M K2SO4 for determinations of dissolved organic carbon (DOC) and dissolved organic nitrogen (DON), using a TIC-TOC analyzer (TOC-VCPH/TOC-VCPN Total Organic Carbon Analyser, Shimadzu, Analytical & Measuring, Instruments Division, Kyoto, Japan). Data on inorganic C were excluded from the analyses, as the values were not detectable and were assumed to be zero. To obtain data on soil nitrate (NO3 −) and ammonium (NH4 +), fresh soil samples were extracted using 2M KCl and analyzed using a flow-injection analysis system (QuikChem® Method 12-107-04-1-B and QuikChem® Method 12-107-06-2-A, nitrate and ammonium, respectively; LACHAT Instruments, HACH, Dublin, Ireland). The nitrate and ammonium analyses were performed only for the topsoil layer (5 to 10 cm) in January, April, July, and October, corresponding to winter, spring, summer, and autumn, respectively. To estimate soil TC and TN stocks, we multiplied the values for soil bulk density with their respective percentage of C and N given by the CHN elemental analyzer, and then expressed the results on an area basis (Mg ha−1).
Soil DNA Extraction and Microbiome Profiling
Soil bacterial community analyses were carried out on 18 samples collected during the summer, due to the poor recovery of DNA of sufficient quality from winter samples. The extraction of DNA from the soil was carried out using the DNeasy PowerSoil Pro kit (Qiagen Ltd., Hathersage Road, Manchester, M13 0BH, United Kingdom) following the manufacturer’s instructions. The DNA concentration (ng µl−1) and absorbance ratios (260:280 nm and 260:230 nm) were quantified by spectrophotometry using an ND-1000 Spectrophotometer (ThermoFisher, 168 Third Avenue, Waltham, MA 02451, USA). Microbiome profiling was carried out by Eurofins Genomics (Konstanz, Germany). The V3-V4 region of the bacterial 16S rRNA gene was amplified using the primers 357-F (TACGGGAGGCAGCAG; Turner et al. Reference Turner, Pryer, Miao and Palmer1999) and 800R (CCAGGGTATCTAATCC; Kisand et al. Reference Kisand, Cuadros and Wikner2002), and resulting amplicons were sequenced using the Illumina MiSeq platform. Amplicon sequences were processed using mothur v. 1.45.3 (Schloss et al. Reference Schloss, Westcott, Ryabin, Hall, Hartmann, Hollister, Lesniewski, Oakley, Parks, Robinson, Sahl, Stres, Thallinger, Van Horn and Weber2009). Sequences smaller than 200 bp, with 1 or more ambiguous nucleotides and homopolymers longer than 8 bp were removed from the dataset. Remaining sequences were aligned against the Silva reference alignment (Quast et al. Reference Quast, Pruesse, Yilmaz, Gerken, Schweer, Yarza, Peplies and Glöckner2013), and those sequences classified as plastid, mitochondrial, archaeal, or eukaryotic or that were unknown at the kingdom level were also removed from the dataset. Chimeric sequences were detected using the vsearch algorithm tool within mothur (Rognes et al. Reference Rognes, Flouri, Nichols, Quince and Mahé2016) and were then also removed. The sequences were classified to genus level in mothur using the built-in Ribosomal Database Project sequence aligner (Schloss Reference Schloss2009). Relative abundances were determined as the abundance of each genus divided by the total number of sequences in a sample.
Determination of the Age of the Invasion
Dating of the age of the invasions was carried out following the methodology of Fennell et al. (Reference Fennell, Gallagher, Vintro and Osborne2014) on four soil cores collected from two different invasive stands at Dooega Beach. This technique is based on the germination of seeds from different soil layers that have been dated using 137Cs. Briefly, we collected soil cores, sliced them into 2-cm disks, put the disks into trays mixed with compost in a greenhouse, and counted the numbers of seeds germinating. The last layer of the soil that had germinating seeds of G. tinctoria was correlated with the radiometric chronology to determine the age of the G. tinctoria invasion and its presence in the studied area. Full details and a critique of the approach are given in Fennell et al. (Reference Fennell, Gallagher, Vintro and Osborne2014).
Statistical Analysis
To compare seasonal differences (i.e., summer and winter) between soil bulk density, DOC, DON, and soil TC and TN stocks among the two treatments, we used general linear mixed-model effects with restricted maximum likelihood, with uninvaded grasslands (GRASS) and areas invaded by G. tinctoria (GUN) as fixed factors and fields as random factors. We used the same approach to check differences between the four seasons (winter, spring, summer, and autumn) regarding the concentrations of ammonium and nitrate for the topsoil layer (5 to 10 cm). ANOVA was applied to check for differences in isotopic compositions. Bonferroni’s post hoc correction was applied after each test, and visual analysis of the residuals was also carried out to ensure normality. These analyses were performed with a significance level of P = 0.05, using SPSS Statistics v. 24 (IBM 2016).
For assessments of changes in the soil microbial community, multivariate statistical analysis was carried out in PRIMER v. 7.0.21 with PERMANOVA+ 1 add-on v. 1.0.1 (Quest Research Ltd., Mahoenui Valley Road, RD3 Albany, Auckland 0793, New Zealand). Potential differences in the microbial populations were also assessed using nonmetric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (PERMANOVA). Microbial diversity was checked using Shannon’s H′ index. Datasets were square-root transformed before their conversion into resemblance matrices based on the Bray-Curtis dissimilarity measure. The NMDS was performed on ranked dissimilarities using the Kruskal fit scheme 1. PERMANOVA tests were done by permutation of residuals under a reduced model using 9,999 permutations, and ANOVA was carried out using SAS v. 9.4 (SAS Institute, Cary, C, USA).
Results and Discussion
Soil Food Web and Gunnera tinctoria
The leaves and roots of the GRASS plants had a similar isotopic composition, with mean (±SE) δ13C and δ15N values of −28.8‰ (0.8) and 3.21‰ (1.1) in leaves and −29.0‰ (0.7) and 3.65‰ (0.3) in roots, respectively (Figure 1A). By contrast, δ15N was highly variable between Gunnera plant organs, spanning from 3.2‰ (0.6) in rhizomes to 6.9‰ (0.5) in leaves, with intermediate values (4.8‰ ± 0.8) in the petiole. Contrary to expectations, there were no differences in δ15N between the grassland vegetation and the G. tinctoria samples, although the latter species can access atmospheric N2 through its symbiosis with Nostoc. No differences in soil δ15N between the two vegetation types were registered either, possibly because of the presence of N-fixing legumes (e.g., Trifolium spp.) in the resident community (Mantoani et al. Reference Mantoani, Sweeney and Osborne2025). There was, however, a large spacing in the C isotope composition between the grass plants and any G. tinctoria plant part (F(1, 13) = 60.69, P > 0.001), because G. tinctoria had δ13C values that were much less negative, ranging from −25.6‰ (0.2) in the rhizome to −24.5‰ (0.5) in leaves (Figure 1A).
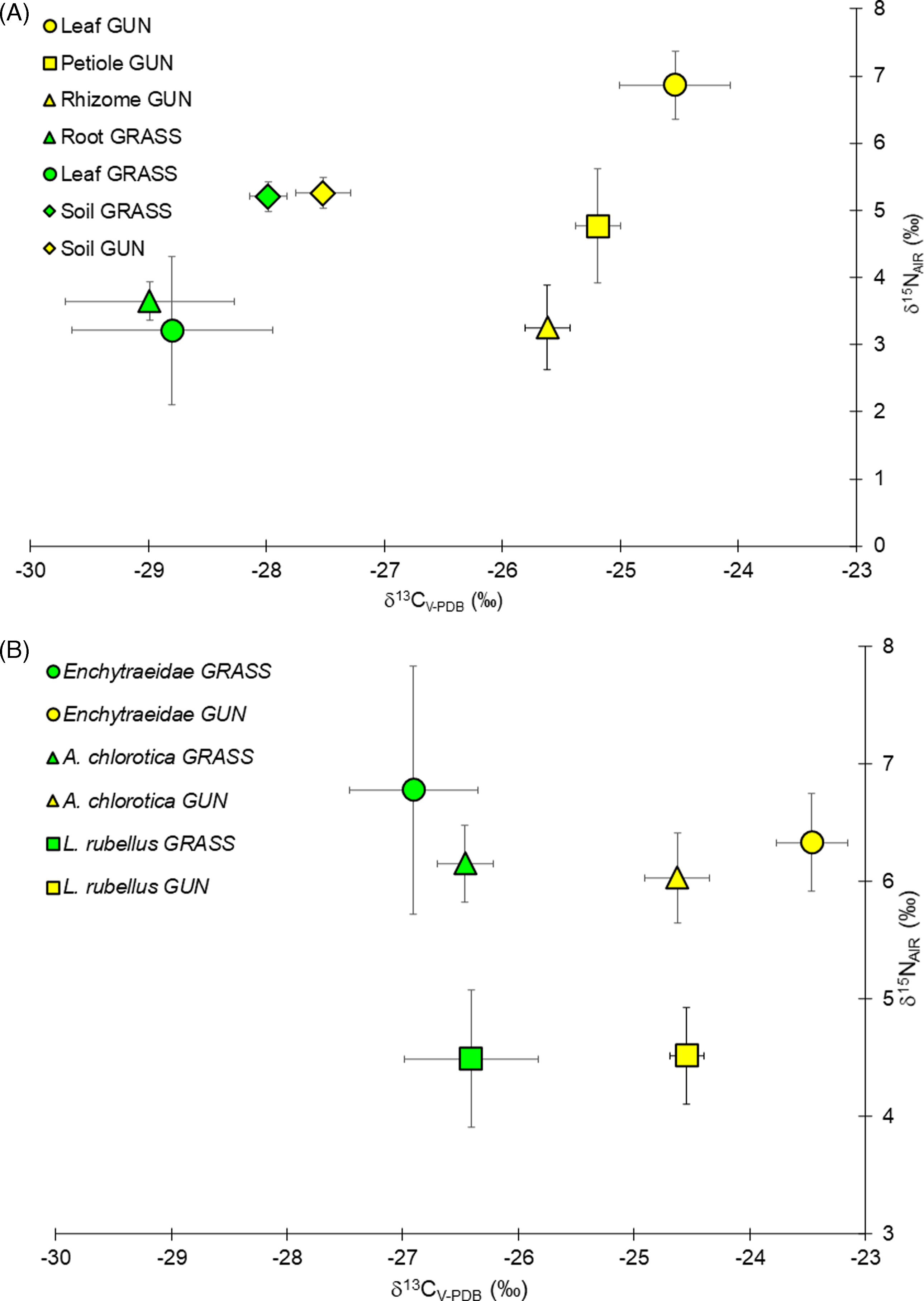
Figure 1. Biplots of the carbon (C) and nitrogen (N) isotope composition of (A) plant parts and soil, and (B) enchytraeid worms and two earthworm species (Allolobophora chlorotica and Lumbricus rubellus) collected on Achill Island, County Mayo, Ireland. GRASS, uninvaded semi-natural grasslands (n = 9, 5, and 6 for earthworms, soil, and plant parts, respectively; mean ± 1 SE); GUN, areas invaded by Gunnera tinctoria (n = 11, 5, and 9 for earthworms, soil, and plant parts, respectively; mean ± 1 SE).
Despite the long site colonization history, the δ13C values of bulk soil organic matter had only shifted marginally from uninvaded grasslands (−28.0‰ ± 0.2) toward less negative G. tinctoria values in invaded areas (−27.5‰ ± 0.2); that is, only a small amount of Gunnera-derived C was evident (Figure 1A). This picture was in stark contrast to the isotopic composition of the detritivorous soil animals, because the δ13C spacing in living plants was clearly reflected in all three taxa of worms (F(2, 27) = 15.31, P = 0.00004), irrespective of feeding mode. Under G. tinctoria, the δ13C of the litter-feeding earthworm L. rubellus had shifted by 1.9‰ toward Gunnera, and in the soil-feeding A. chlorotica, it had shifted by 1.8‰. In the tiny enchytraeid worms, the δ13C had even shifted by 3.4‰ (Figure 1B).
The results of the isotopic analysis demonstrated that G. tinctoria is driving changes in the earthworm and enchytraeid community through trophic mechanisms, with an increased (i.e., 20-fold higher litter production) and higher-quality (i.e., lower C:N ratio) food supply at its base, in comparison to uninvaded areas (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022). There are many examples of invasive plant species that are shown to increase litter inputs (Ehrenfeld Reference Ehrenfeld2003; Tamura and Tharayil Reference Tamura and Tharayil2014; Wolkovich et al. Reference Wolkovich, Lipson, Virginia, Cottingham and Bolger2010), and many have a higher biomass productivity than native species (Bossdorf et al. Reference Bossdorf, Auge, Lafuma, Rogers, Siemann and Prati2005; Sakai et al. Reference Sakai, Allendorf, Holt, Lodge, Molofsky, With, Baughman, Cabin, Cohen and Ellstrand2001), with potentially similar consequences for the soil fauna. In the invaded areas we sampled, there was a marked effect of G. tinctoria on earthworm populations, with more than a 2-fold increase in biomass and an almost 3-fold increase in earthworm abundance (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022), reinforcing the idea that invasive plants can significantly modify the soil fauna (Pearson et al. Reference Pearson, Minor, Robertson and Clavijo-McCormick2024; Reinhart and Callaway Reference Reinhart and Callaway2006; Zhang et al. Reference Zhang, Li, Wu and Hu2019). While top invasive predators might disrupt trophic food webs (Wainright et al. Reference Wainright, Muhlfeld, Elser, Bourret and Devlin2021), plant invasions that produce large amounts of biomass and displace native plants, as G. tinctoria does (Gioria and Osborne Reference Gioria and Osborne2013; Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020), appear to have the opposite effect, and favor some animal species (i.e., earthworms).
Measured above-ground litter accumulation at the invaded sites peaked at 9.9 Mg dry weight (DW) ha−1 (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022). Using a range of reported feeding rates for litter-feeding earthworms (Curry and Schmidt Reference Curry and Schmidt2007) of between 10 and 50 mg DW g−1 live earthworm biomass d−1 (and assuming 300 active d yr−1 at the study site), we can estimate that the resident litter-feeding earthworm population (about 60 g biomass m−2) can consume between 1.8 and 9.0 Mg DW litter yr−1. These estimates suggest that the litter-feeding earthworm populations under G. tinctoria can consume a large proportion, if not most, of the litter produced in a year. Furthermore, the large spacing in δ13C observed in our study between G. tinctoria and grassland species of about 4‰ allowed us to infer the C sources of detritivorous worms in invaded areas. Under living G. tinctoria, the C isotopic composition of the two earthworm species and the much smaller enchytraeid worms had clearly shifted towards that of the invasive plant and away from that of the bulk soil organic matter. This suggests that G. tinctoria drives the soil food web by providing enhanced C inputs. Both litter- and soil-feeding worms species exhibited this shift, reflecting the fact that old, recalcitrant soil organic matter is of minor importance as a C source (Curry and Schmidt Reference Curry and Schmidt2007). The isotope data nicely complement the earthworm population data from Mantoani et al. (Reference Mantoani, González, Sancho and Osborne2020), because they demonstrate a trophic link as a mechanism by which invasive plants can alter below-ground communities.
Soil Bacterial Community and Gunnera tinctoria Invasions
In total, 105 bacterial genera were detected in uninvaded plots, and 121 were present in invaded areas. Alpha diversity was similar in both areas, with a Shannon H′ index of 2.55 in uninvaded areas and 2.27 in invaded areas. Based on the 20 most abundant genera in each area, the composition of the soil bacterial community under invaded stands was significantly different from that in uninvaded grasslands (P = 0.0001) at the genus level. Microbiome analysis revealed that, at the genus level, the composition of the soil bacterial community in invaded areas was significantly distinct from that in uninvaded grasslands (P = 0.0001) based on the most abundant genera. Soil depth also significantly affected bacterial community dynamics (P = 0.0032, Supplementary Table 1), with pair-wise analyses confirming that the community in invaded areas was different from that in the top soil layer of uninvaded areas (5 to 10 cm; t = 2.83, P = 0.014), for the middle layer (20 to 25 cm; t = 2.22, P = 0.01), and the deepest soil layer analyzed (50 to 55 cm; t = 2.21, P = 0.02). The NMDS plot (Figure 2) shows these differences and confirms that the soil bacterial community under invasive stands differed significantly from that found in the uninvaded plots, with the difference being maintained at each soil depth.
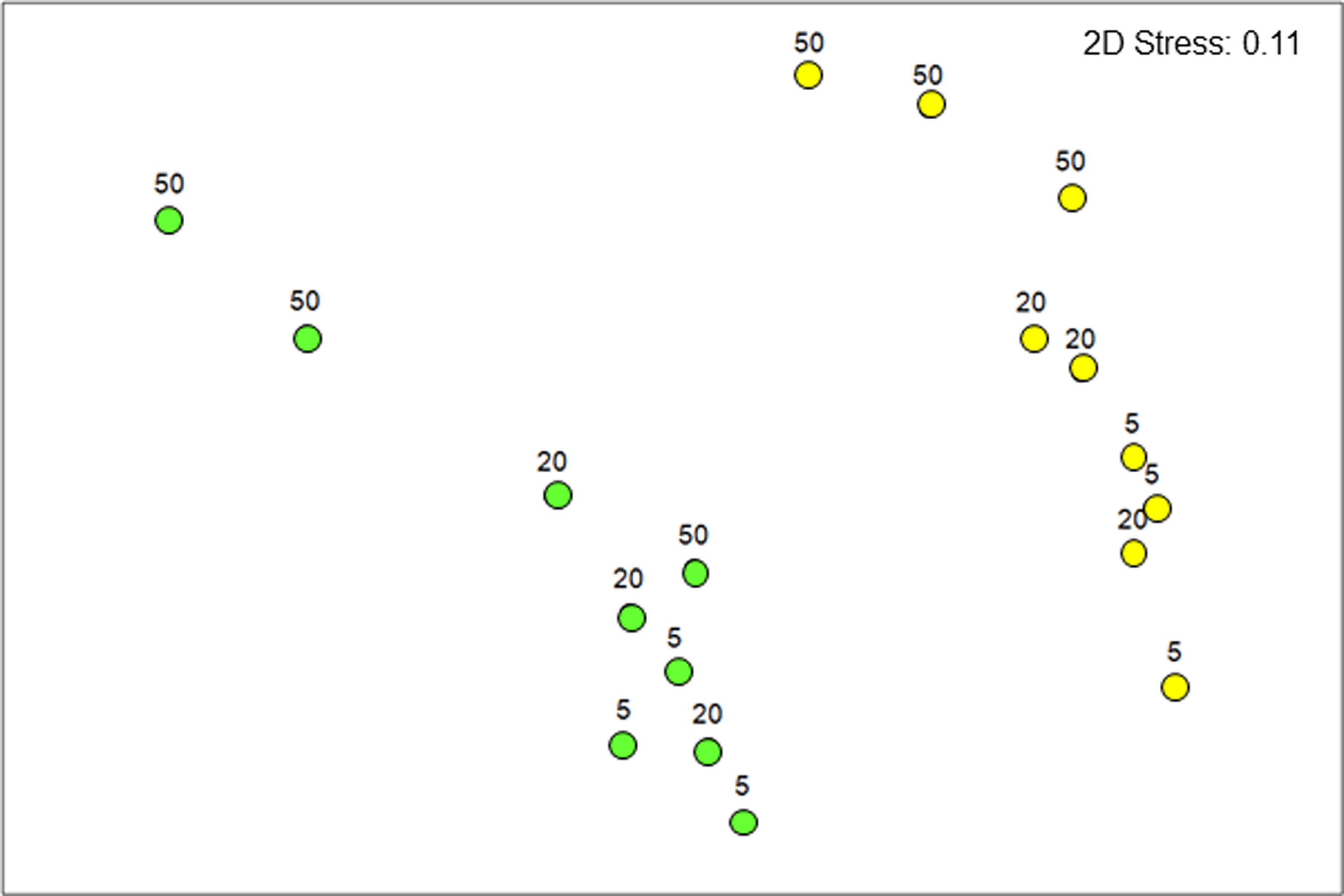
Figure 2. Nonmetric multidimensional scaling (NMDS) plot for the soil bacterial community present in uninvaded grasslands (GRASS, green dots) and areas invaded by Gunnera tinctoria (GUN, yellow dots) (n = 9) on Achill Island, County Mayo, Ireland. Soil depth is indicated by numbers above the symbols (5 = 5 to 10 cm; 20 = 20 to 25 cm; 50 = 50 to 55 cm). Note: this analysis considers only soil samples collected during the summer due to the low retrieval of DNA in the winter.
The dominant genus in both areas was Bacillus, accounting for approximately 22% of the genera detected (Table 1). Genera belonging to Acidobacteria groups 1, 2, and 3 were also dominant in both areas, accounting for a combined ca. 35% of the genera detected in uninvaded areas and ca. 23% of the genera in invaded areas. The relative abundance of the genus Nitrospira was significantly higher under invasive stands than in uninvaded grasslands (P = 0.008), accounting for 7.32 ± 1.38% and 2.94 ± 0.46% of the sequences detected, respectively. Six bacterial genera (Aciditerrimonas, Acidobacteria Gp7, Conexibacter, Defluviicoccus, Pseudonocardia, and Rhodomicrobium) were among the 20 most abundant genera in uninvaded areas only. Conversely, a further six genera (Acidobacteria Gp5, Acidobacteria Gp13, Hyphomicrobium, Nocardioides, Sporosarcina, and genera belonging to WS3 genus incertae sedis) were among the most abundant genera in invaded plots only (Table 1). Changes in the abundance of methanotrophs were also found, with the Methylocystaceae family almost 3-fold higher and Methylobacter 4-fold lower in invaded areas in comparison to uninvaded grasslands. However, these changes were not significant due to the high variation between individual replicates.
Table 1. Relative abundance (%) of the 20 most abundant bacterial genera in uninvaded semi-natural grasslands (GRASS) and areas invaded by Gunnera tinctoria (GUN) ± 1 SE a
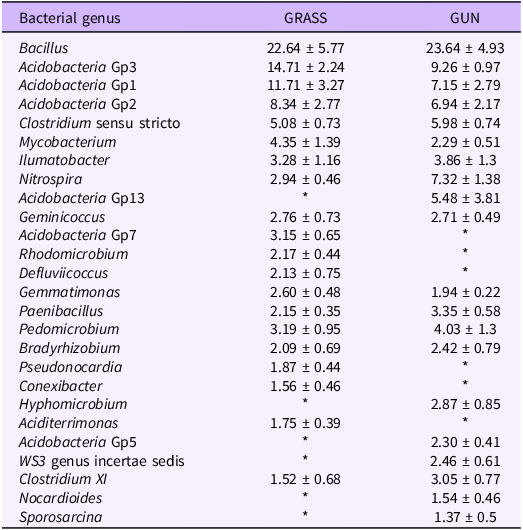
a An asterisk (*) indicates that a genus was not among the top 20 most abundant bacterial genera in a particular treatment.
Differences in soil bacterial communities under invasive stands have been reported previously (Gibbons et al. Reference Gibbons, Lekberg, Mummey, Sangwan, Ramsey and Gilbert2017; McTee et al. Reference McTee, Lekberg, Mummey, Rummel and Ramsey2017; Reinhart and Callaway Reference Reinhart and Callaway2006), and our study reinforces the idea of invasive plants as drivers of soil bacterial communities. Although the majority of the 26 most abundant soil bacteria genera found in invaded areas were not significantly different from those in the uninvaded grasslands, there was a reduction n the abundance of six bacteria genera (Aciditerrimonas, Acidobacteria Gp7, Conexibacter, Defluviicoccus, Pseudonocardia, and Rhodomicrobium). In addition, the abundance of a further six soil bacteria genera (Acidobacteria Gp5, Acidobacteria Gp13, Hyphomicrobium, Nocardioides, Sporosarcina, and WS3 genus incertae sedis) increased in invaded areas (i.e., appeared in the top 20). Interestingly, the presence of this plant invader more than doubled the abundance of Nitrospira spp., which is usually involved in the oxidation of nitrite to nitrate (Daims and Wagner Reference Daims and Wagner2018).
Although the reason(s) for these differences are not known, both enchytraeids and endogeic earthworms obtain a significant proportion of their essential amino acids from soil bacteria (Larsen et al. Reference Larsen, Pollierer, Holmstrup, D’Annibale, Maraldo, Andersen and Eriksen2016). Given the large increase in earthworms in invaded areas, particularly the endogeic forms (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022), this could have resulted in a reduction in some bacterial genera. Many Acidobacteria (group 7) are thought to play a key role in the C, N, and sulfur biogeochemical cycles (Kalam et al. Reference Kalam, Basu, Ahjmad, Sayyed, El-Enshasy, Dailin and Surian2020), and this could reflect a reduced role in invaded areas. Similarly, the generally significant role of the Actinomycetota in organic matter breakdown (Bao et al. Reference Bao, Dolfing, Guo, Chen, Meng, Li, Lin and Feng2021) may be associated with the increased involvement of earthworms in decomposition processes in invaded areas. The reduction in Rhodomicrobium spp. in invaded areas could be because the abundance of this phototrophic bacterium is limited by the intense shade provided by the G. tinctoria canopy (Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020). The increases observed for the other bacteria genera, including Nitrospira spp., are more difficult to explain, but could be related to a general increase in easily metabolizable substrates provided by the decomposition of the large amounts of high-quality litter and/or are directly or indirectly associated with the more abundant earthworm populations.
A growing body of evidence suggests that Nitrospira is also involved in the COMAMMOX process, that is, complete oxidation of ammonia to nitrate by a single organism (Daims et al. Reference Daims, Lebedeva, Pjevac, Han, Herbold, Albertsen, Jehmlich, Palatinszky, Vierheilig, Bulaev and Kirkegaard2015; van Kessel et al. Reference van Kessel, Speth, Albertsen, Nielsen, op den Camp, Kartal, Jetten and Lücker2015). COMAMMOX Nitrospira are thought to be widely distributed (Shi et al. Reference Shi, Jiang, Wang, Wang and Zhu2020), with some studies suggesting that COMAMMOX may be the dominant nitrification pathway in soils and other environments (Hu et al. Reference Hu, Yuxiang, Yao, Wang, Zheng, Xi and Hu2021; Zhao et al. Reference Zhao, Huang, He, Zhou, Wang, Dang, Wang and Zheng2019). In the present study, ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) were poorly represented, with only 15 sequences related to Nitrosomonas (AOB) and 13 sequences to Nitrobacter (NOB) detected. No reads related to other AOB such as Nitrosococcus, Nitrosospira, or Nitrococcus (AOB) were found. Approximately 13,500 sequences relating to Nitrospira were present, suggesting that complete ammonia oxidation may occur underneath invasive stands.
There is evidence that the soil bacterial genera that are promoted by G. tinctoria invasions, including Nitrospira, are linked to a higher N availability (Xu et al. Reference Xu, Wang, Li, Jiang, Zhou, Ding, Zong, Ling, Zhang and Lu2020), and nitrate and DON accumulated in the topsoil layer in the current study. The factors underpinning these effects are often associated with higher-quality litter inputs and the specific association between different invasive plants and their rhizosphere organisms, with potentially wide-ranging impacts for the whole ecosystem (Broadbent et al. Reference Broadbent, Orwin, Peltzer, Dickie, Mason, Ostle and Stevens2017; Ehrenfeld Reference Ehrenfeld2003; Hawkes et al. Reference Hawkes, Wren, Herman and Firestone2005). The increase in soil pH during the summer under invasive stands (Mantoani and Osborne Reference Mantoani and Osborne2022) can influence soil TC/TN stocks (Jiao et al. Reference Jiao, Shi, Han and Yuan2016) and nitrification/denitrification processes, leading to higher soil nitrate levels (Šimek and Cooper Reference Šimek and Cooper2002), which was observed in the current study. All these effects can lead to modifications in the composition and activity of the soil bacterial community (Che et al. Reference Che, Zhao, Zhou, Jia and Shen2015), favoring genera/species that are linked to high N availability in invaded areas. In addition to the observed changes in bacteria associated with soil N transformations, we also found evidence for alterations in the abundance of methanotrophs, indicating that there may be wider impacts on the soil microbial community than those associated with N-related metabolic pathways.
Soil Physiochemical Properties and Gunnera tinctoria Invasion
Soil bulk density increased with depth (F(2, 48) = 10.15, P < 0.001), but was not significantly different between invaded and uninvaded areas (F(2, 48) = 0.003, P = 0.997) for any season studied (Supplementary Table 2). No differences in DOC were found between the two treatments (F(2, 48) = 0.067, P = 0.935; Figure 3A). The levels of DON, however, decreased with soil depth in both treatments (F(2, 48) = 80.38, P < 0.001) and were higher in the G. tinctoria plots in the near-surface layer (5 to 10 cm; F(1, 48) = 8.99, P = 0.004; Figure 3B). Differences in DON were up to 50% higher (38.06 mg N kg soil−1) in invaded areas (CI = 58.88, 93.94 and 96.94, 131.99, uninvaded grasslands and invaded areas, respectively).
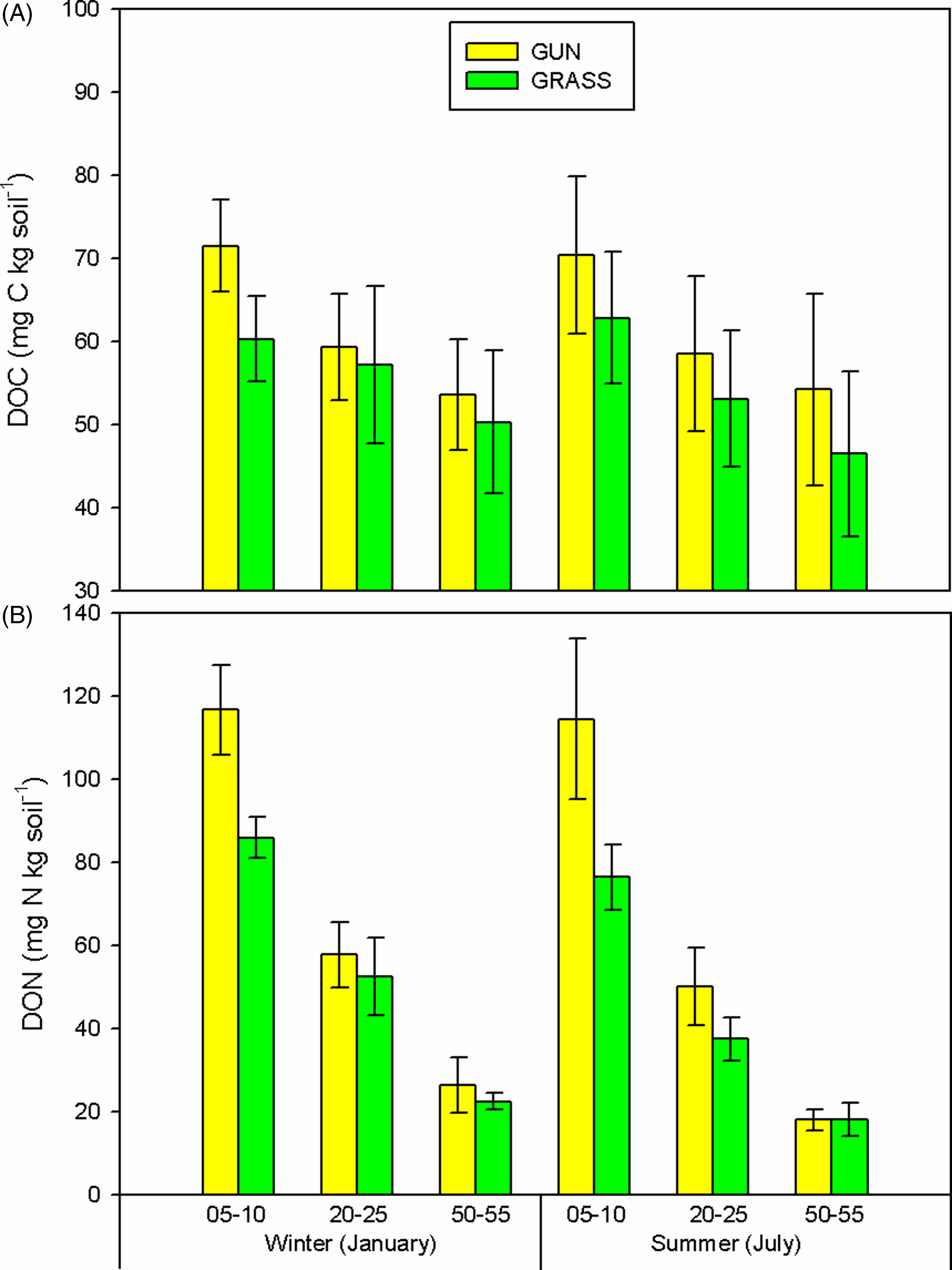
Figure 3. Seasonal variation in (A) dissolved organic carbon (DOC, mg C kg soil−1) and (B) dissolved organic nitrogen (DON, mg N kg soil−1) during 2016, according to the three soil depths analyzed (5 to 10, 20 to 25, and 50 to 55 cm), on Achill Island, County Mayo, Ireland (n = 5; mean ± 1 SE). GRASS, uninvaded semi-natural grasslands; GUN, areas invaded by Gunnera tinctoria.
Together with an increase in DON in the near-surface layers under invasive stands, we also found an increase in nitrate (F(3, 24) = 6.65, P = 0.002) throughout the year, which elevated the soil concentrations by 6.40 mg N kg soil−1 (95% Confidence Interval, CI = 0.25, 12.56) in summer and by 8.73 mg N kg soil−1 (95% CI = 2.58, 14.89) in autumn, in comparison to uninvaded plots (Figure 4B). Concomitantly, there was a reduction in ammonium levels (F(3, 24) = 12.63, P < 0.001) throughout the year (Figure 4A), with uninvaded grasslands showing a decrease of 11.42 mg N kg soil−1 (95% CI = 4.40, 18.45) and invaded plots a decrease of 9.19 mg N kg soil−1 (95% CI = 2.17, 16.22). There was an increase in soil TC stocks with soil depth (F(2, 48) = 3.51, P = 0.038), but no significant differences were found between treatments (F(1, 48) = 0.293, P = 0.591) or seasons (F(1, 48) = 0.0004, P = 0.984; Supplementary Figure 1A). No differences in soil TN stocks were found among treatments (F(1, 48) = 0.222, P = 0.640), seasons (F(1, 48) = 1.42, P = 0.239), or soil layers (F(2, 48) = 0.205, P = 0.816; Supplementary Figure 1B).
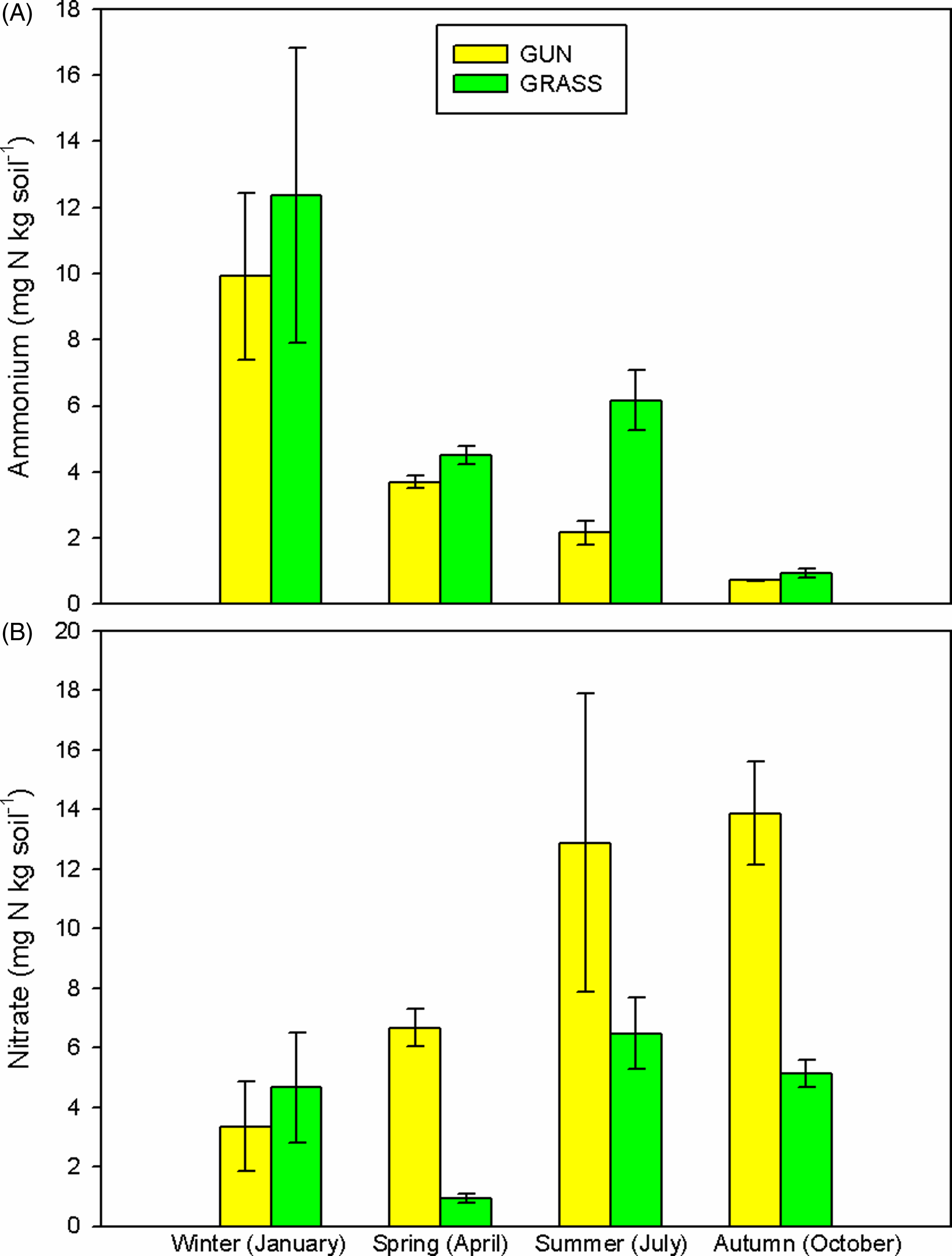
Figure 4. Seasonal variation in (A) ammonium (mg N kg soil−1) and (B) nitrate (mg N kg soil−1) in the topsoil layer (5 to 10 cm) throughout 2016, on Achill Island, County Mayo, Ireland (n = 5; mean ± 1 SE). GRASS, uninvaded semi-natural grasslands; GUN, areas invaded by Gunnera tinctoria.
It is important to note that the increased numbers of earthworms underneath the canopy of G. tinctoria could have an impact in altering soil C and N, as described before (Bohlen et al. Reference Bohlen, Scheu, Hale, McLean, Migge, Groffman and Parkins2004), and could also contribute to shaping the soil bacterial community, which in turn could alter the soil nutrient balance. As illustrated previously (de Graaff et al. Reference de Graaff, Adkins, Kardol and Throop2015), interactions between earthworms and soil bacteria contribute to soil TC and TN stocks. Such interactions could augment the loss of nutrients under invasive stands, reducing any potential differences in soil physiochemical attributes between uninvaded and invaded sites and indicating that higher plant productivity does not necessarily translate into higher soil TC and TN stocks. Consequently, invasive alien plants that produce a higher-quality (low C:N ratio) litter with faster decomposition rates may not necessarily increase C stocks, due to a loss of soil C, possibly through microbial priming effects (Tamura and Tharayil Reference Tamura and Tharayil2014). This could reflect the situation in invaded areas where the previous recalcitrant grassland litter inputs have been replaced by more accessible and faster decomposing material supplied by G. tinctoria (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022). It further suggests that invasive plants may not always induce any major changes in soil properties (Stefanowicz et al. Reference Stefanowicz, Stanek, Nobis and Zubek2017), if at the same time they modify the soil biota (Pearson et al. Reference Pearson, Minor, Robertson and Clavijo-McCormick2024; Stefanowicz et al. Reference Stefanowicz, Stanek, Nobis and Zubek2016).
Significance of the Age of the Invasion and the Generality of the Impacts
Based on the seed germination results from different soil layers, dated using 137Cs, the age of the invasion was estimated at 107 yr and 49 yr for the first and the second soil cores, respectively. The deepest soil layers at which seed germination occurred were 25 and 27 cm for the first and the second cores, respectively. The chronologies were also consistent with the 137Cs profiles for both soil cores. Thus, the date for the first appearance of significant G. tinctoria populations that were examined in the current study was approximately 110 yr and 50 yr, for the first and second soil cores, respectively. One of the problems associated with many assessments of the ecosystem impact of introduced plants is the uncertainty about the duration of the invasion, which can have a major impact on the results obtained (Flory et al. Reference Flory, Bauer, Phillips and Clay2017).
Based on the estimates of the age of the different populations in this (49 to 107 yr) and a previous study (70 to 100 yr; Fennell et al. Reference Fennell, Gallagher, Vintro and Osborne2014), the populations we studied had an average age of >80 yr. The variability in the estimates could be due to variations in colonization, growth, and development, which could vary with site and environmental conditions. While these results indicate that long-lived (>49 yr) G. tinctoria populations have an altered detrital food chain, it is unclear how long it takes from the initial establishment of introduced populations to produce these modifications and how reversable they might be (i.e., presence of legacy effects). Previous results involving the removal or herbicide treatment of mature G. tinctoria populations have shown that earthworm numbers can return to within 30% of those in uninvaded areas within 1 yr (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022), confirming that the increase in earthworm populations is largely dependent on the current year’s litter production.
These results show that once a significant pool of litter is produced, continuous annual inputs are required to maintain the worm populations and associated alterations in the detrital food chain. Of particular significance is the development of a large rhizome, as annual above-ground productivity is high once this is formed (Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020). A conservative estimate of how long this takes, based on personal observations, could be on the order of 10 to 20 yr, an age that is significantly less than the populations examined in the current study, when sufficient litter may be present to result in a significant change in the worm population, but further work would be required to confirm this. Moreover, the generality of these effects is also unclear. In New Zealand, invasive Scotch broom (Cytisus scoparius (L.) Link, a leguminous N-fixing shrub) was associated with larger populations of (undetermined) Oligochaeta (Pearson et al. Reference Pearson, Minor, Robertson and Clavijo-McCormick2024). On the other hand, common rhododendron (Rhododendron ponticum L.) invasions led to the disappearance of most of the earthworm populations in two independent studies in the United Kingdom and Ireland (Clare Reference Clare2016; Melody and Schmidt Reference Melody and Schmidt2021). While the reason(s) for these differences are not known, the lower-quality litter produced by R. ponticum (C:N = 45:1) compared with G. tinctoria (C:N = 17:1) may be a factor (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022). Similarly, the lower C:N ratio in the uninvaded grassland areas used in the current study (C:N = 29:1) may also contribute to the lower earthworm populations independent of differences in litter production.
The evidence presented in this work expands on previous studies by identifying trophic mechanisms that explain why G. tinctoria invasions can have wide-ranging and contrasting impacts on ecosystems, including detrimental or neutral effects (Mantoani et al. Reference Mantoani, González, Sancho and Osborne2020; Mantoani and Osborne Reference Mantoani and Osborne2022) as well as beneficial or positive effects (Mantoani et al. Reference Mantoani, Alhakami, Fearon, Gioria, Schmidt and Osborne2022; Mantoani and Osborne Reference Mantoani and Osborne2021a). The current study shows that G. tinctoria invasions can result in a shift in the soil biota and modifications in the detrital food chain, with an increased role for earthworms in decomposition processes. Overall, these results indicate the complexity of impacts that an invasive species may have on ecosystems and their biotic processes, highlighting the need for more work that encompasses a range of invasive species to assess the generality of these results.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/inp.2025.12
Data availability
Data will be made available upon request to the corresponding author.
Acknowledgements
The authors are grateful for the support received from local landowners and Mayo County Council. We also thank Eugene Sherry and Cristina Motta Pechin, who gave exceptional help in the field; Darragh Furey for technical assistance and sample preparation; and Marie and Martin McGreal for their hospitality and lovely accommodation.
Funding statement
MCM was supported by the Brazilian National Council for Scientific and Technological Development (CNPq; grant number 205031/2014-5).
Competing interests
The authors declare no conflicts of interest.








