Management Implications
Schinus terebinthifolia (Brazilian peppertree) has been introduced to subtropical and coastal regions of North America, including Florida, Hawaii, and parts of Texas and California, and in other parts of the world, including Australia and South Africa. Current management practices are costly and limited in their reach and permanency, necessitating repeated applications of chemical and mechanical control efforts. Biological control of invasive weeds using insects has proven to be an effective and safe tactic that can reduce the need for frequent herbicide applications. In 2019, release of a biological control agent, the thrips Pseudophilothrips ichini, to target S. terebinthifolia commenced in Florida to enhance control efforts. The success of P. ichini, like that of all biological control agents, depends on a variety of factors in its new environment. This study focused on understanding interactions between S. terebinthifolia, P. ichini, and a naturally occurring stem gall–inducing fungus (Cophinforma sp.), and the potential impacts to agent success against the target plant. We confirmed that galling on S. terebinthifolia plants does not affect the reproductive output of P. ichini or the damage it inflicts on the plant. This study provides evidence to suggest the presence of galls would not impede the efficacy of the biological control agent.
Introduction
Brazilian peppertree (Schinus terebinthifolia Raddi (Zona Reference Zona2015), Sapindales: Anacardiaceae) is a large woody shrub that grows quickly and outcompetes native vegetation in invaded environments. This aggressive growth has made it one of the top invasive species management priorities in Florida (Rodgers et al. Reference Rodgers, Pernas and Hill2014). To address this, the biological control agent Pseudophilothrips ichini (Thysanoptera: Phlaeothripidae), henceforth referred to as “thrips,” was approved by the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service (USDA-APHIS) for field releases in 2019. Since then, thrips have established populations at various sites across Florida (Wheeler et al. Reference Wheeler, Minteer, Rohrig, Steininger, Nestle, Halbritter, Leidi, Rayamajhi and Le Falchier2022). The thrips larvae and adults feed gregariously on flushing S. terebinthifolia stems and leaves, often killing new stem tips (Wheeler et al. Reference Wheeler, Silverson, Dyer and Mc Kay2016). The biological control efficacy of the thrips has been demonstrated in laboratory (Harlow et al. Reference Harlow, Harms and Schad2023; Manrique et al. Reference Manrique, Diaz, Erazo, Reddi, Wheeler, Williams and Overholt2014; Wheeler et al. Reference Wheeler, Jones, Broggi and Halbritter2018) and outdoor garden plot (Halbritter et al. Reference Halbritter, Kariuki, Wheeler, Rayamajhi, Minteer and Read2024a) studies, but more research is needed to understand its performance in field settings in the invaded range and how it will interact with other organisms utilizing S. terebinthifolia as a food source.
Understanding insect–plant–pathogen interactions can generate beneficial outcomes for classical biological control of introduced weeds. Fungal pathogens may influence herbivorous insects by altering the tissues of the host plant, causing resistance, consuming nutrients, and producing toxins (Clay Reference Clay1987). Mutualistic interactions between fungi and insects enhance plant herbivory, whereas mutualism between fungi and plants usually decreases herbivory (Clay Reference Clay1987). There is no clear unifying model for insect–plant–fungus interactions. It is likely insects independently develop behaviors to either prefer or reject fungus-infected plants, influenced by environmental conditions in each system (Raman and Suryanarayanan Reference Raman and Suryanarayanan2017).
Synergistic insect–pathogen interactions have been observed in specific systems. For instance, Aphthona spp. (Coleoptera: Chrysomelidae) feeding on leafy spurge (Euphorbia esula L.) facilitate the penetration of soilborne pathogens such as Rhizoctonia spp., Fusarium spp., and Pythium spp., reducing weed density within invaded regions (Caesar Reference Caesar2003, Reference Caesar2011). Similarly, larvae of Neochetina eichhorniae (Coleoptera: Curculionidae), used as biocontrol agents of invasive waterhyacinth [Eichhornia crassipes (Mart.) Solms], create entry points for the fungal pathogen Acremonium zonatum, which reduces photosynthesis in leaves and ultimately accelerates plant death (Charudattan et al. Reference Charudattan, Perkins and Littell1978). Evaluating candidate biological control agents for potential insect–pathogen synergies or conducting survival analyses of target weeds with combined insect and pathogen approaches could improve weed management outcomes while minimizing costs and environmental risks (Caesar Reference Caesar2003).
Endophytic fungi residing within plant tissues exhibit diverse interactions with their hosts. While some endophytes form mutualistic relationships, others act as latent pathogens that become virulent under stress conditions. These fungi can modulate plant defense mechanisms and nutrient profiles, potentially altering herbivore behavior (Raman et al. Reference Raman, Wheatley and Popay2012; Rodriguez et al. Reference Rodriguez, White, Arnold and Redman2009). Insect-induced galls often serve as nutrient sinks and may also support fungal colonies. Gall midges (Illiciomyia yukawai), for instance, transport fungi into plant tissues, creating a mutualistic relationship that benefits both the insect and the fungus (Kobune et al. Reference Kobune, Kajimura, Masuya and Kubono2012).
Fungal galls on S. terebinthifolia stems at different heights and intensities have been noted since at least 1979 and were initially attributed to the fungal species Sphaeropsis tumefaciens (Botryosphaeriaceae) (Marlatt and Ridings Reference Marlatt and Ridings1979). We noted these morphologically similar galls on S. terebinthifolia in both nursery stock at our thrips-rearing facility and in numerous field sites across southeastern Florida, and we began recording gall sightings in 2020. Recently, a molecular study has identified this gall-inducing organism to be the member of a fungal genus Cophinforma (Halbritter et al. Reference Halbritter, Rayamajhi, Madeira, Leidi, Telmadarrehei and Minteer2024b). Thus far, nothing is known about whether interactions between this Cophinforma sp. and thrips will be synergistic, additive, neutral, or antagonistic with respect to damage inflicted to S. terebinthifolia and consequent impacts on biocontrol efficacy.
We tested the hypothesis that Cophinforma-induced galls affect the fecundity and damage potential of thrips using a two-choice laboratory study with potted nursery plants experimentally infected with the fungus in a previous study (Halbritter et al. Reference Halbritter, Rayamajhi, Madeira, Leidi, Telmadarrehei and Minteer2024b). Our findings aim to shed light on the potential interactions between this Cophinforma sp. and thrips and their implications for biological control of S. terebinthifolia.
Materials and Methods
Plant Preparation
Schinus terebinthifolia saplings were grown from seed in 3.8-L plastic pots at the USDA-Agricultural Research Service Invasive Plant Research Laboratory (IPRL) in Fort Lauderdale, FL, USA; maintained outdoors with daily irrigation, routine pruning, fertilization, and insecticide treatments; and prepared for inoculation with the Cophinforma fungus as in Halbritter et al. (Reference Halbritter, Rayamajhi, Madeira, Leidi, Telmadarrehei and Minteer2024b). Briefly, 11-mo-old plants were moved into a glasshouse after being treated with systemic and topical fungicides and were maintained as above for 3 mo before inoculation with the fungus. Each of the 24 plants had five inoculation points made by abrading the bark on woody sections of different branches and fastening mycelial mats of fungus to the wounds using sterilized cotton gauze held in place with masking tape. Twelve control plants were treated in the same manner, but only water was added to the gauze. After 1 mo, the gauze was removed, and plants continued to be maintained as above for 3 more months followed by a final pruning and insecticide treatment occurring roughly 1 mo before setting up the cage experiment described in the following section.
Cage Setup and Maintenance
By the time of the caged experiment, plants were 18 mo old and 75- to 115-cm tall, with roughly 10 to 20 main stems each bearing at least 15 cm of flushing stem tissue, and an additional 10 to 40 live axillary meristems in the canopies of each plant. With these plants, we conducted a two-choice test in which 40 thrips adults were added to each of six rectangular acrylic rearing cages (Halbritter et al. Reference Halbritter, Rayamajhi, Wheeler, Leidi, Owens and Cogan2021). Each cage contained one control plant (ungalled) and one inoculated with Cophinforma, a symptomatic plant with visible galls. Plants were paired in a manner to maximize the similarity of multiple demographic variables (see the following section). Adult thrips roughly 5 d after pupal emergence (likely mated and ready for oviposition by that time [DAH, personal observation]) were harvested from our mass production colony at the IPRL, and groups of 40 were placed in plastic 185-ml (50-dram) snap-cap vials, each with a cutting of S. terebinthifolia. Roughly equal numbers of males and females were selected and remained in vials for 24 h. Each cage replicate for the two-choice test received one vial of 40 thrips, which was placed on the bottom of the cage between the two plants and opened, allowing thrips to disperse and make their plant selections. This also allowed us to gain insights on how thrips may move between galled and ungalled plants in field settings where plants are intermingled. As a control measure to account for tip mortality caused by the fungus alone, six cages were set up in the same manner, but thrips were not added. All 12 cages, regardless of whether they contained thrips, had Sphagnum L. moss on top of the soil in each pot, and layered paper towels were added to the bottoms of the cages for thrips pupation. Plants were watered every other day by adding 250 ml of water to saucers in which the pots were placed. Lighting was provided by banks of four 30-W fluorescent grow lights (sunlite® F54T4/865/HO, sunlite, Brooklyn, NY) on a 14:10 light:dark photoperiod. There were four light banks, with one bank positioned 22 cm above three cages. Laboratory conditions were maintained at 22 to 24 C and 45% to 60% relative humidity, while microclimates within the cages ranged from 22 to 26 C and 54% to 76% relative humidity. To control for imbalances in light and temperature in the laboratory, we moved each cage two positions to the right and rotated cages 180° daily for the first week (during which time oviposition was likely to be the greatest [Halbritter and Wheeler Reference Halbritter and Wheeler2021]), then weekly for the remainder of the study. The experiment concluded after the last of the F1 thrips adults had finished emerging, which was after 40 d.
Plant and Thrips Data Collection
Before plant pairing and thrips introduction, each plant had the following demographic variables measured: number of live meristems (both apical and axillary); combined length of live, flushing stem tissue; number of dead meristems (both apical and axillary); combined length of dead, necrotic stem tissue; and number and size of galls. The length of live, flushing stem tissue was the distance from the apex of the meristem to the point along the stem where lenticels began to appear and the stem hardened. The length of dead, necrotic stem tissue was the distance from the apex of the dead meristem to the midpoint where blackened and shriveled stem tissue transitioned to living stem tissue. Galls were measured by their length along the stem, width across the stem, and height protruding from the stem.
Nine days after adult thrips introduction, all plants were monitored approximately every other day to document the number of F1 larvae on each plant inside each cage. Larvae were left undisturbed and were free to move between plants, and we considered daily occupancy as a proxy for plant preference. Thrips counts ceased after F1 larval numbers began to drop below roughly 10% of the maximum number seen, as temporal emergence in lab colonies is typically unimodal. At that point (40 d after thrips introduction), all plants were removed from their boxes, and the same demographic measurements were taken. Plants were returned to their cages and the experiment concluded after the last of the F1 adult thrips were collected. Some surviving adult F0 thrips may have been included in counts, but this was assumed to be equivalent for all replicates and a small percentage of the F1 totals.
Foliar Nitrogen
Undamaged, flushing leaves were collected once from all plants 13 d after thrips introduction but before F1 larval numbers began to climb, allowing plants time to acclimate to cage environments but before significant damage commenced. At that point, all plants were 18.5 mo old. For galled plants, leaf samples were taken from galled stems distal to the gall. For ungalled plants, leaf samples were taken from randomly selected stems. Three leaf samples, each roughly 5 cm in length, were sampled from each plant. Leaves were dried in an oven at 60 C for at least 1 wk and then ground to a powder. Percent nitrogen was determined using a CHN analyzer (Perkin Elmer Series II CHNS/O Analyzer 2400, Norwalk, CT). Briefly, 2 mg of sample was packed into a tin cup and up to eight samples were run in series. Between series, a tomato leaf standard (NIST 1573a Tomato Leaves, Gaithersburg, MD) was used to verify accuracy of the machine.
Statistical Analyses
To account for plants having different numbers of stem tips and some dead tips at the start of the experiment, the percentage of dead tips ([dead/(dead + live)] × 100) was calculated for each plant at the beginning and end of the experiment. Due to some plants starting out with no dead tips, we subtracted the pre- from posttreatment dead tip percentages from each plant for analyses. Gall-specific necrosis was noted before thrips inoculation and monitored over the course of the study. The final length of the gall necrotic stems was subtracted from the total necrosis to get thrips-induced necrosis. To determine the final percentage of live, flushing stem material that succumbed to thrips-induced necrosis ([total length of necrosis/(total length of necrosis + total length of remaining live flush)] × 100), we subtracted the pre- from posttreatment thrips-induced necrosis percentages from each plant for analyses.
We used a linear mixed-effects model to determine how the dead tips response was impacted by thrips presence, gall presence, and the interaction between the two. A generalized linear mixed-effects model was used for stem necrosis, given the data better fit a gamma distribution. For these two analyses, cage was treated as the random effect. Analyses of deviance (type III Wald chi-square tests) were used to obtain the test statistics for the fixed effects and their interaction. When examining the effect of gall presence on larval F1 thrips numbers, we calculated the number of thrips per centimeter of starting flushing stem material to control for variable amounts of initial flushing stem material. The effect of gall presence on the number of F1 larval thrips per centimeter of starting flushing material was assessed with a t-test using the maximum number of thrips seen on each plant as the response variable. We assumed an ellipsoid shape to calculate the volume of each gall: ([(height/2) × (width/2) × (length/2)] × (4/3) × π). A t-test was then used to determine the effect of thrips presence on the percent change in gall volume. Finally, we used a t-test to determine the effect of galling on foliar percent nitrogen. We used R software v. 4.3.1 (R Development Core Team 2023) for all analyses.
Results and Discussion
Dead Stem Tips
Dead stem tips caused by galls at the start of the experiment only accounted for 2.8% of dead stem tips counted at the end of the experiment. Regardless of gall presence, thrips had a significant impact on the proportion of dead stem tips by the end of the study (χ2 = 15.022, P < 0.001), with more dead tips in the cages with thrips added intentionally (Figure 1). However, galling had no influence on the number of dead tips resulting from thrips feeding at the end of the study. There was also no significant interaction between thrips and gall presence impacting stem tip mortality.
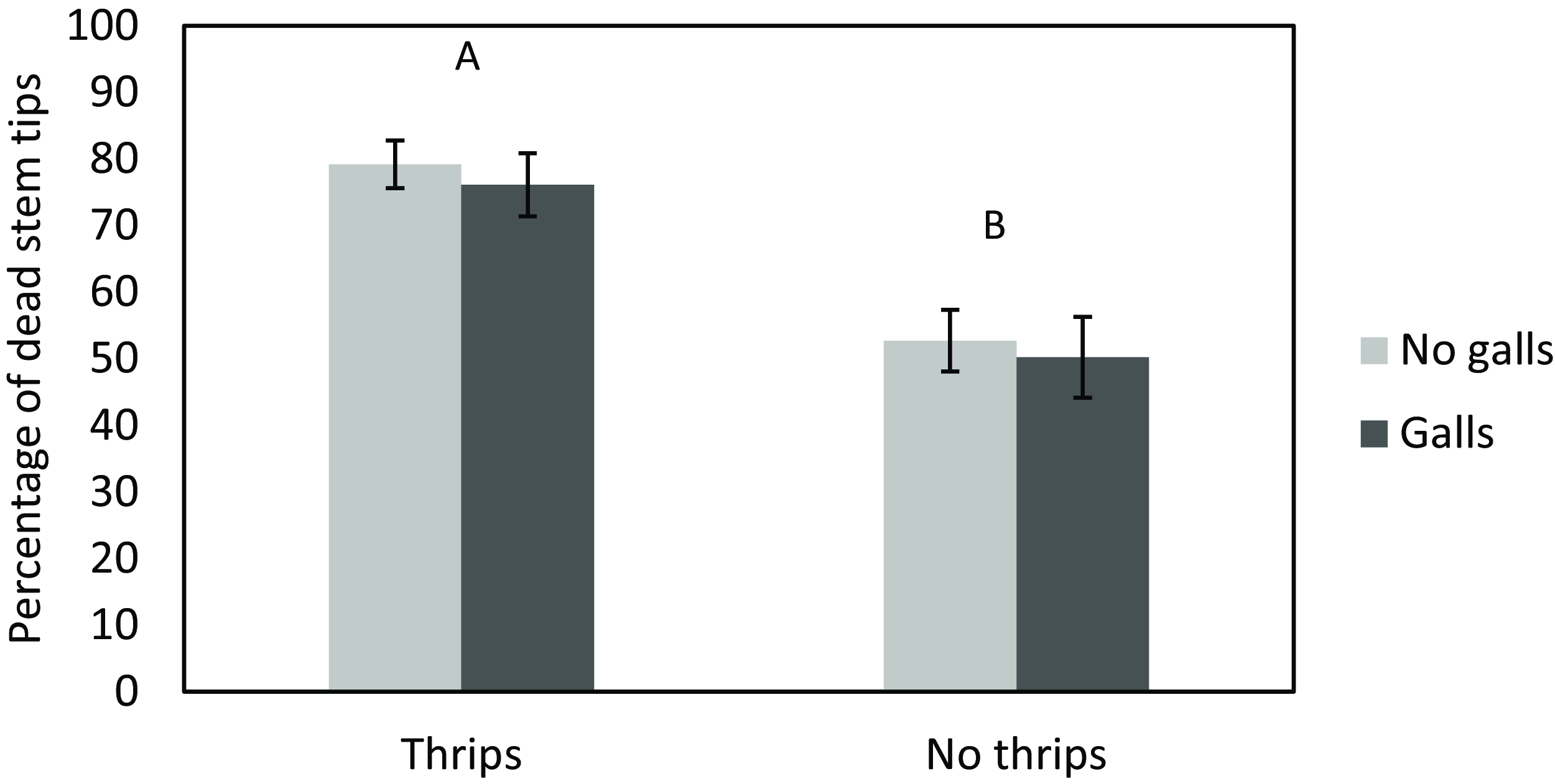
Figure 1. Assessment of dead stem tips on Schinus terebinthifolia due to thrips (Pseudophilothrips ichini) feeding and the impact of stem galls induced by Cophinforma sp. The bar pairs represent thrips added (left) and no thrips added (right), with two levels of galling within pairs (no galls in light gray and galls in dark gray). Different uppercase letters above bars indicate significant differences between thrips and no thrips treatments with P < 0.05. Error bars represent the standard error of the mean.
Extent of Stem Necrosis
Total stem necrosis caused by galls only accounted for 15.6% of the total length of necrosis. Regardless of gall presence, thrips had a significant impact on the percentage of flushing stem length that succumbed to necrosis by the end of the study (χ2 = 20.293, P < 0.001), with greater necrosis on plants in cages into which thrips were added (Figure 2). However, galling had no influence on the difference in the post- minus pretreatment percentage of flushing stem length succumbing to necrosis as a result of thrips feeding at the end of the study. There was also no significant interaction between thrips and gall presence impacting stem necrosis.
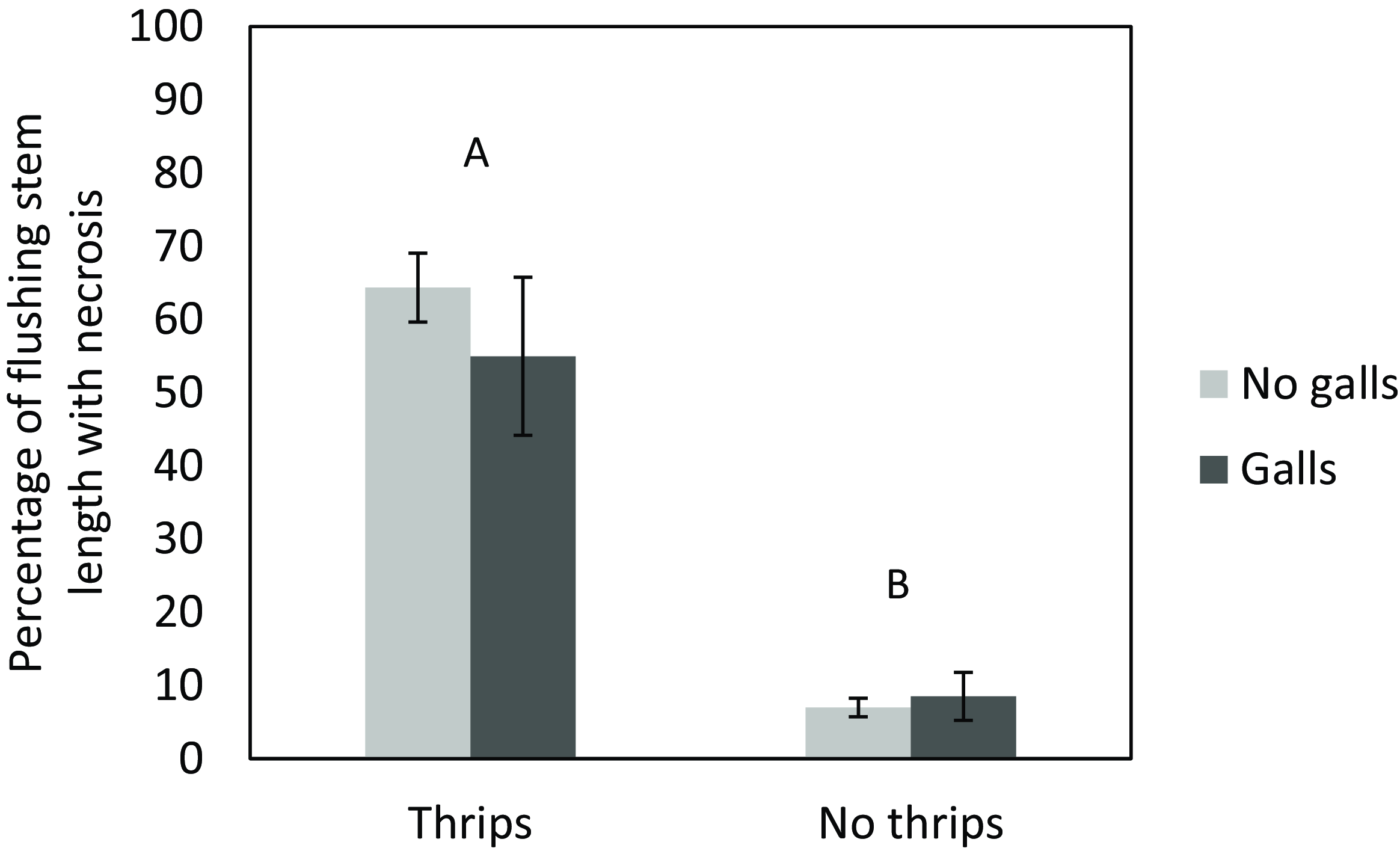
Figure 2. Assessment of the impact of stem galls induced by Cophinforma sp. on the extent of Schinus terebinthifolia stem tip necrosis induced by the thrips Pseudophilothrips ichini. The bar pairs represent thrips added (left) and no thrips added (right), with two levels of galling within pairs (no galls in light gray and galls in dark gray). Different uppercase letters indicate significant differences between thrips and no thrips treatments. There were no within-cage differences for the galling treatment. Error bars represent the standard error of the mean.
Thrips Numbers
Thrips numbers per centimeter of initial flushing stem length were not impacted by whether the plant had galls, with an average maximum daily density of 0.53 ± 0.07 and 0.46 ± 0.09 thrips cm−1 flush for ungalled and galled plants, respectively. The average number of F1 adults harvested from each of the six thrips-infested cages at the end of the study was 926 ± 64. Over the 1-mo period of F1 larval monitoring, counts per plant gradually rose, peaked at roughly 2 wk, then gradually declined as larvae began to pupate. The average total number of intruding adults and their F1 larvae removed from each of the six uninfested control cages over the course of the study was 90 ± 14. These were loose thrips in the lab that found their way into the control cages, laid eggs, and were removed every other day.
Gall Growth
The number of galls induced on inoculated plants ranged from 2 to 5 with an average of 3.75 galls per plant. Final gall sizes ranged from 4.22 mm by 2.65 mm by 1.78 mm to 14.39 mm by 8.1 mm by 6.65 mm, with average (±SE) dimensions of 11.45 ± 0.51 mm by 6.00 ± 0.33 mm by 4.05 ± 0.32 mm. In the presence of thrips, gall growth significantly slowed (t = −2.419, P = 0.041), with only a 0.24 ± 6.39% increase in cumulative gall volume for thrips-infested plants compared with 29.74 ± 10.39% increase in cumulative gall volume on thrips-free (not intentionally infested) plants (Figure 3).
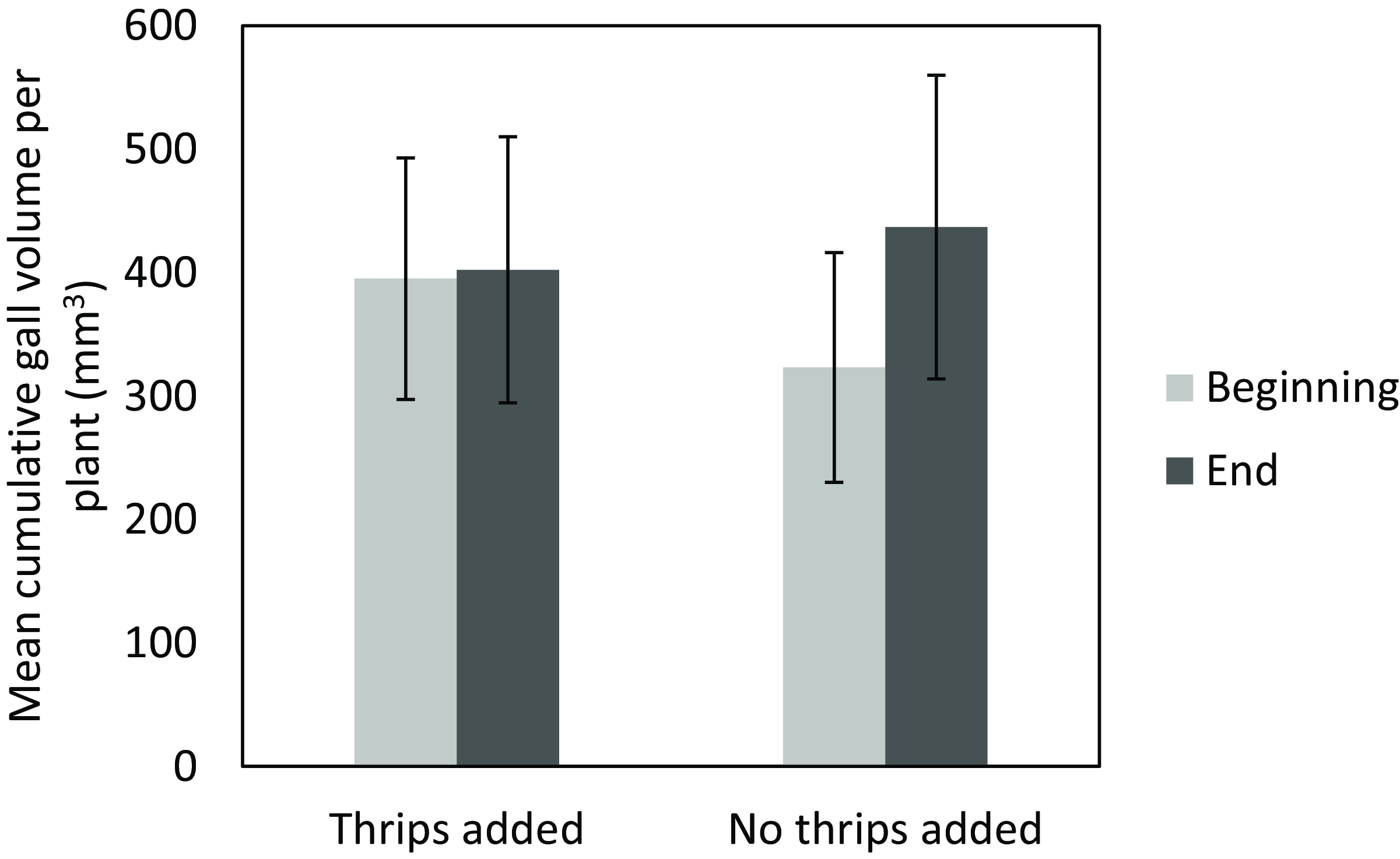
Figure 3. Assessment of the impact of thrips presence on the growth of Schinus terebinthifolia stem galls induced by Cophinforma sp. The two bar pairs compare cumulative gall area at the beginning and end of the experiment with and without the thrips Pseudophilothrips ichini added, which covers the time for one cohort of the thrips to complete development from egg to adult. Statistical analysis was conducted on the percent changes between beginning and end data. Error bars represent the standard error of the mean.
Foliar Nitrogen
The percent foliar nitrogen in flushing leaves on stems emerging from galls (2.84 ± 0.05%) did not differ significantly from the percent foliar nitrogen in flushing leaves on random stems on ungalled plants (3.05 ± 0.13%; t = 1.359, P = 0.1896).
Synthesis
We demonstrate here that the presence of initial-onset Cophinforma sp.-induced galls on S. terebinthifolia saplings has no impact on the damage thrips causes to the plants and no effect on the number of F1 larvae produced from such plants, nor is there a thrips preference for galled or ungalled plants. Jami et al. (Reference Jami, Marincowitz, Durán, Slippers, Abad, Chen and Wingfield2022) reported there was no correlation or clear pattern between species in the Botryosphaeriaceae family (seven species of Lasiodiplodia, four species of Neofusicoccum, Endomelanconiopsis endophytica, and Cophinforma atrovirens) and insect damage occurrence on the same trees. However, Moral et al. (Reference Moral, Morgan, Trapero and Michailides2019) cited studies confirming hemipteran feeding damage facilitates disease caused by Botryosphaeriaceae infection in nut crops, and Jami et al. (Reference Jami, Slippers, Wingfield and Gryzenhout2013) suggested cerambycids may facilitate fungal disease symptoms in Acacia karroo Hayne by stressing branches. In the present study, P. ichini limited gall growth, likely due to the rapid loss of leaves and drop in photosynthesis. However, plants later manifested more severe galling.
In the days after the F0 adults were added to the thrips cages, we noted which plants tended to have more adults, and this switched regularly between the galled and ungalled plants. Although F1 larvae can be mobile, they tend to remain near their oviposition site, at least initially. Older, second instar larvae will wander to new, undamaged flush, and this could have been the main driver of movement between plants as stems died back later in the study and thrips searched for new material. Also, the more conspicuous second instar larvae are more likely to be detected, and this likely contributed to the greatest thrips counts on each plant. Therefore, considering larvae could also make a choice of plant after their parents made an oviposition choice and seeing no consistent differences in plant occupancy, even as L1 larvae were first being detected, results suggest thrips do not have a plant preference during either of their feeding stages.
In other systems, fungal phytopathogens have been shown to manipulate both the foraging behavior and life cycle of insect herbivores (Franco et al. Reference Franco, Túler, Gallan, Gonçalves, Favaris, Peñaflor, Leal, Moura, Bento and Silva-Filho2021). By the time thrips were introduced to infected plants, changes in plant chemistry with respect to an induced response to infection have likely already taken place, as resistance gene expression in plants can occur within minutes to hours following infection (Shen et al. Reference Shen, Liu, Li, Wang, Xu, Chen, Xing and Zheng2017). Initial colonization by endophytic fungi thickens the plant cell wall, inducing resistance against pathogen attacks (Sharma et al. Reference Sharma, Malthankar and Mathur2021). The host resistance to pathogens indirectly impacts arthropod communities and their herbivore feeding behaviors. Both pathogens and herbivores also depend on the species and genotype of their host plants, which influence multitrophic interactions (Busby et al. Reference Busby, Lamit, Keith, Newcombe, Gehring, Whitham and Dirzo2015). We saw some hints that foliar nitrogen was lower in tips emanating from galls, but the difference was not significant. While thrips have been seen feeding on flushing stems emerging from galls on mature S. terebinthifolia in the field (Figure 4), nothing is known about whether their performance is impacted or whether they have feeding preferences for flush emerging from galls within larger trees in the field with greater progression of infection severity. We propose further work to survey thrips on galled and ungalled trees and to survey thrips on flush within infected trees emerging from galls and flush emerging from healthy branches.

Figure 4. Pseudophilothrips ichini larvae (in yellow circle) feeding on Schinus terebinthifolia flushing stem material growing out of a stem gall in the field.
When considering gall performance, thrips feeding pressure appeared to have a negative impact on the galls due to arrested gall growth. In certain conditions, like early-stage infection but with catastrophic thrips damage ongoing, thrips could outcompete the fungus. Competition has been demonstrated between spider mites (Tetranychus urticae) and a vascular wilt fungus (Verticillium dahliae) (Karban et al. Reference Karban, Adamchak and Schnathorst1987). Thrips-mediated reduction of the photosynthetic capability of S. terebinthifolia will subsequently reduce the availability of metabolites needed by the plant for continued expansion of galls in its attempt to compartmentalize the fungus. Another study showed that a different S. terebinthifolia agent, Calophya latiforceps (Hemiptera: Calophyidae), can significantly reduce the photosynthetic output of S. terebinthifolia (Prade et al. Reference Prade, Diaz, Vitorino, Cuda, Kumar, Gruber and Overholt2016), and this is likely the case here with thrips. In our study, up to 95% of the stem tips were killed and the plants were significantly defoliated. Any leaves not damaged directly by the thrips died as stem necrosis expanded past leaf petioles, resulting in flagging, where dead, drooping leaves remain on a dead branch. In the field, we usually do not see this level of damage, at least not at new release sites. Plants attacked by thrips in the field would likely still have enough leaf area to provide photosynthates to divert to gall growth. However, we have not measured galls on trees in the field before and after thrips establishment, and this warrants future monitoring.
The interplay between the relatively new thrips agent and the already naturalized fungus is complex and may be dependent on the timing and severity of damage inflicted by both species. Under minimal care in the glasshouse, we retained the infected plants from this study after thrips were removed, and after 6 more months, galls were present on sections of the plant that were not wounded for inoculation points. At that point, stem tips emerging from galls began dying back considerably, in some cases killing the plant at 16 mo postinoculation (Figure 5). While previously regarded by Marlatt and Ridings (Reference Marlatt and Ridings1979) as insufficient to control the plant, the gall fungus is clearly capable of proliferating and causing severe damage under the right circumstances.

Figure 5. Potted Schinus terebinthifolia at 16 mo after inoculation with Cophinforma sp. and one generation of Pseudophilothrips ichini feeding damage. The plant has since died following proliferation of stem galling beyond inoculation sites and multiple stem tips that emerged from the galls dying back.
If this naturalized fungus has potential to gain ground on S. terebinthifolia when the plant is stressed, then other naturalized herbivores may be able to as well. We have noted considerable scale and aphid infestations on S. terebinthifolia at sites suffering from heavy thrips damage, with sooty mold (Dothideales) also prevalent. Past field surveys have identified several species of native and naturalized herbivores on S. terebinthifolia in Florida (Bhattarai et al. Reference Bhattarai, Diaz, Manrique, Turechek, Buss, Stange and Overholt2017; Cassani Reference Cassani1986; Cassani et al. Reference Cassani, Maloney, Habeck and Bennett1989), yet these species have also been insufficient to control the plant in its invaded range. Our work here provides support for research in weed biocontrol systems to reach beyond investigating direct agent impacts: How can the added pressure of reuniting plants with their specialized natural enemies change plant defenses to herbivores/pathogens already present in the invaded range that may not have exerted sufficient control before agents were added? Our study is the first report of the interaction among endophyte-induced fungal galls, an S. terebinthifolia thrips agent, and S. terebinthifolia.
Acknowledgments
We are grateful for the work of Laura Cash, Jenna Owens, Carly Cogan, and Nicholas Vallone to produce, maintain, and prepare potted S. terebinthifolia plants for this experiment. We thank the two anonymous reviewers for their time and effort in providing constructive feedback leading to this article.
Funding statement
This research was partly funded by the U.S. Department of Agriculture– Agricultural Research Service (USDA-ARS) and the Comprehensive Everglades Restoration Plan, a cooperative agreement between the USDA-ARS Invasive Plant Research Laboratory, South Florida Water Management District, and U.S. Army Corps of Engineers.
Competing interests
The authors declare no conflicts of interest. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity employer and provider.








