Management Implications
Chrysanthemoides monilifera subsp. monilifera (syn. Osteospermum moniliferum subsp. moniliferum; boneseed) is a shrub native to the southwestern and southern coasts of South Africa. In Australia, C. monilifera subsp. monilifera was introduced in the 1850s and has spread extensively in the southeastern states of Victoria, South Australia, and Tasmania. The introduction of C. monilifera subsp. monilifera to Western Australia is thought to have happened almost a century later, and spread remains far more restricted. After C. monilifera subsp. monilifera was classified as a Weed of National Significance in Australia in 1999, detailed plans for its management were developed, drawing on a synthesis of available data at the time. This coordinated effort resulted in two detailed literature reviews being produced in 2006 and 2008. Since this time, research and management on C. monilifera subsp. monilifera has continued to generate new insight. In South Australia, containment lines are being applied, and more effective biological control may improve management outcomes. In Western Australia, a recent synthesis of past management has revealed that eradication remains a realistic target. All of these programs would benefit from an updated synthesis of relevant knowledge. Our review has brought together new information from the past two decades of research on the ecology, biology, and management of C. monilifera subsp. monilifera, with the specific goal of improving the chance of eradicating C. monilifera subsp. monilifera in Western Australia. A lack of detailed understanding of key ecological issues relating to management remains, including whether or not it is an obligate outcrossing taxon and the impacts of seed dispersal vectors in its non-native range. In contrast, understanding of its germination requirements and allelopathic effects and the effectiveness of management options, including fire and herbicides, have advanced. Regarding classical biological control, our review has revealed plausible explanations as to why the existing agents have not resulted in broadly effective management. Management of C. monilifera subsp. monilifera in southeastern Australia and New Zealand’s South Island remains focused on containment where possible, some localized extirpations, and minimizing impact. Invasions elsewhere in the world remain largely unmanaged, but at the same time lack documented evidence of rapid invasion. Our synthesis supports retaining eradication as the management goal for C. monilifera subsp. monilifera in Western Australia via a mixed approach of manual removal, herbicide, and controlled burns where logistics permit.
Introduction
Boneseed [Chrysanthemoides monilifera subsp. monilifera (L.) Norl.; syn. Osteospermum moniliferum subsp. moniliferum L., Asteraceae], is a perennial shrub native to the southwestern and southern coasts of South Africa (Weiss et al. Reference Weiss, Adair, Edwards, Winkler and Downey2008). In Australia, C. monilifera subsp. monilifera was first recorded as an introduced plant in Sydney in 1852, from “MacLeay’s garden” Melbourne, in 1858 (and subsequently grown in Melbourne suburbs as a garden plant), from Adelaide in 1892 at the West Terrace Cemetery, and from Ulverstone, Tasmania, in 1931 (Weiss et al. Reference Weiss, Adair and Edwards1998). At the time, this shrub was mostly cultivated as an ornamental garden plant, but there may have been deliberate naturalization attempts in western Victoria in the You Yang Ranges and to stabilize coastal sand dunes between Nelson and Portland, Victoria (Weiss et al. Reference Weiss, Adair and Edwards1998).
Currently, C. monilifera subsp. monilifera is widely distributed in southern Australia (Brougham et al. Reference Brougham, Cherry and Downey2006). Its distribution in southeastern Australia covers an area from the Eyre Peninsula in South Australia to the Victorian–New South Wales border and extends from the coast to a significant way inland in some areas. Extensive infestations occur in South Australia’s Mount Lofty Ranges and Murraylands and Victoria’s Mornington Peninsula, Bellarine Peninsula, and the You Yang Ranges. In New South Wales, scattered infestations are present along the coast from Newcastle on the Central Coast to Moruya in the south. The majority of infestations are in the Sydney region, extending west into the Blue Mountains (Brougham et al. Reference Brougham, Cherry and Downey2006). In Tasmania, C. monilifera subsp. monilifera is concentrated around the fringes of inhabited areas and is mainly restricted to coastal and estuarine areas (Brougham et al. Reference Brougham, Cherry and Downey2006). The disjunct distribution in Western Australia is more restricted in its range and abundance, found across multiple populations between Albany and Perth (Batchelor et al. Reference Batchelor, Yeoh, Bell, Campos, Ota, Richetti and Webber2023b, Reference Batchelor, Scott and Webber2024).
Chrysanthemoides monilifera subsp. monilifera prefers winter rainfall regions, where it is found in a wide range of vegetation communities, including coastal dunes, estuarine areas, heath, mallee, woodland, and dry and wet sclerophyll forest (Brougham et al. Reference Brougham, Cherry and Downey2006). Chrysanthemoides monilifera subsp. monilifera occurs on a range of soil types but does not tolerate water-logged soils (Muyt Reference Muyt2001). Seedlings emerge during winter, reaching reproductive maturity in the second year of growth. Flowering occurs from August to October, with fruiting following during September to November. Flowers are protandrous, with seeds usually produced by allogamy (Weiss et al. Reference Weiss, Adair, Edwards, Winkler and Downey2008). There is 1 seed per fruit, up to 6 seeds per inflorescence, and up to 50,000 seeds per plant, every year (Weiss et al. Reference Weiss, Adair and Edwards1998). In Australia, birds are the primary dispersal vectors, although most seeds fall beneath the plant and either enter the seedbank or are consumed by rodents or ants. Seed longevity is highly variable and likely depends on local context, ranging from at least 3 yr (Weiss Reference Weiss1984) to more than 8 yr (French et al. Reference French, Ashcroft, Panetta, Raghu and Cherry2024), 8.5 yr (Briden and McAlpine Reference Briden and McAlpine2012), and 9 yr (L McMillan, personal communication). Mature plants are estimated to live 10 to 20 yr (Muyt Reference Muyt2001).
Chrysanthemoides monilifera subsp. monilifera’s impact as a weed is most severe in natural ecosystems, and its presence is associated with the decline of flora and fauna in southeastern Australia. Grassy woodland, valley grassy forest, and lowland forest vegetation communities in Victoria are vulnerable to C. monilifera subsp. monilifera invasion, where dense infestations eliminated most native ground flora and prevented virtually all overstory regeneration (Muyt Reference Muyt2001). Dense, continuous canopy cover for C. monilifera subsp. monilifera has been recorded in areas of the You Yang Ranges (Adair and Holtkamp Reference Adair and Holtkamp1999), directly threatening the endangered orchid (Pterostylis truncata Fitzg.) (Adair et al. Reference Adair, Morley and Morin2012; Bramwells Reference Bramwells2003). The local extirpation of some 40 indigenous plant species within these ranges has been largely attributed to the local C. monilifera subsp. monilifera infestation (Blood Reference Blood1987; Thomas et al. Reference Thomas, Possingham and Roush2005). Moreover, removal of C. monilifera subsp. monilifera in a defined 109-ha area has coincided with an overall increase in koalas (Phascolarctos cinereus) and an expansion of their use of this habitat (Duffy Reference Duffy2020).
At the national level, C. monilifera subsp. monilifera (as well as bitou bush [Chrysanthemoides monilifera subsp. rotunda (DC.) J.C. Manning & Goldblatt; syn.: Osteospermum moniliferum subsp. rotundatum (DC.)]) was classified as a Weed of National Significance (WoNS) in 2000, due to the significant impacts it has on natural environments (ARMCANZ and ANZECC 2000). A national strategy was drafted for its management attempting to “arrest the spread and minimise the impact of C. monilifera subsp. rotunda and C. monilifera subsp. monilifera in natural ecosystems” (ARMCANZ and ANZECC 2000). The National Bitou and Boneseed Management Group was formed to implement the goals in the strategy. The strategy was revised in 2012 by the Australian Weeds Committee to progress the legacy of achievements by stakeholders under the previous strategy.
Unlike the C. monilifera subsp. monilifera invasion in the southeast of Australia, populations in Western Australia are often reasonably discrete and appear slow to spread. Some populations are also in areas where local conditions are well suited to detailed monitoring and large-scale management interventions. Chrysanthemoides monilifera subsp. monilifera in Western Australia became a declared plant in 1979 under the Agriculture and Related Resources Protection Act, 1976–1978 (Government Gazette of Western Australia, No. 4, 1980). In 2006, C. monilifera subsp. monilifera in Western Australia was reclassified as a plant not to be introduced to the state, with existing populations targeted for eradication (initially P1/P2, now Category C2). The current category prevents any sale, trade, or movement. Surveillance and management since that time has varied in effectiveness, particularly since 2013, when funding from the national WoNS program ceased (Batchelor et al. Reference Batchelor, Scott and Webber2022).
There is a strong likelihood that a more coordinated, consistent, and systematic approach to surveillance could deliver greatly improved outcomes for C. monilifera subsp. monilifera management in Western Australia, as well as in other places where the plant is an introduced invader. To prepare for such management, two deliverables are required. First, an aggregated synthesis of past management and control efforts is required, detailing present and past population demographics for the weed. Until recently, this has not existed for Western Australia, because C. monilifera subsp. monilifera management has been done by multiple agencies without enduring overall coordination and has not been consistent in either space or time. A synthesis of these data as well as a full assessment of C. monilifera subsp. monilifera populations across the state has recently been completed (Batchelor et al. Reference Batchelor, Scott and Webber2024). This insight will transform the ability of land managers in Western Australia to know where and when to act.
Second, an updated review of the literature covering C. monilifera subsp. monilifera management is essential to leverage existing understanding and identify knowledge gaps. This knowledge will help to inform how to act, particularly in regard to improving the efficiency and effectiveness of management. The reviews of Brougham et al. (Reference Brougham, Cherry and Downey2006) and Weiss et al. (Reference Weiss, Adair, Edwards, Winkler and Downey2008) provided a comprehensive guide to the ecology and biology of C. monilifera subsp. monilifera and its management options in Australia as was best known at that time. The Brougham et al. (Reference Brougham, Cherry and Downey2006) review comprised six sections, providing detailed insight on ecology, biology, control and post-control restoration, and monitoring. It also featured case studies of C. monilifera subsp. monilifera control from South Australia, Tasmania, and Victoria. While there is strong overlap in content between the two publications, the Weiss et al. (Reference Weiss, Adair, Edwards, Winkler and Downey2008) paper provides greater detail on the taxonomy, distribution (actual and potential), plant growth and development, dispersal and population dynamics, and legislative status.
Since these reviews, C. monilifera subsp. monilifera has remained a focus for much research in Australia and elsewhere. While most of this work has focused on C. monilifera subsp. monilifera as an introduced weed, further context from the native range can also provide relevant guidance for refining management. Here we review the past two decades of research on the ecology, biology, and management of C. monilifera subsp. monilifera to update the earlier work of Brougham et al. (Reference Brougham, Cherry and Downey2006) and Weiss et al. (Reference Weiss, Adair, Edwards, Winkler and Downey2008) with the specific goal of guiding more efficient and effective management of C. monilifera subsp. monilifera in Western Australia specifically and elsewhere more generally.
Methods
We took two approaches to assembling content for this review. First, we consulted an extensive literature collection, including “gray” literature, that we have acquired through active searching for any publications on Chrysanthemoides over the past 20 yr (i.e., from when we started working on this genus; Scott Reference Scott1996). Second, further literature on the biology, ecology, and management of C. monilifera subsp. monilifera since 2008 was systematically identified through keyword searches of Web of Science (webofscience.com), Google Scholar (scholar.google.com), and the standard Google search engine (google.com) using the following search strings: boneseed, Chrysanthemoides, Chrysanthemoides monilifera, Osteospermum moniliferum. This last name reflects a more recent taxonomic interpretation of Chrysanthemoides (synonymized in Osteospermum; Sadler et al. Reference Sadler, Parker, Verboom, Ellis, Jackson, van Zyl, Manning and Bergh2022) that has been accepted by the Plants of the World Online database and iNaturalist (POWO 2024) but is yet to be accepted by the Australian Plant Name Index published by the Council of Heads of Australasian Herbaria (https://chah.gov.au). For the purposes of this review, we have retained the use of Chrysanthemoides monilifera subsp. monilifera, based on the currently accepted name in the Australian Plant Census database (https://www.anbg.gov.au/cpbr/program/hc/hc-APC.html). Where relevant and to provide specific context, we referenced literature from before 2008. However, we have not sought to make this a thorough overview of all previous work on C. monilifera subsp. monilifera, given the earlier reviews covering this literature in detail (Brougham et al. Reference Brougham, Cherry and Downey2006; Weiss et al. Reference Weiss, Adair, Edwards, Winkler and Downey2008). While this review focuses on C. monilifera subsp. monilifera, we also include literature on the closely related C. monilifera subsp. rotunda where it aids in the understanding of C. monilifera subsp. monilifera management.
Results and Discussion
History of Chrysanthemoides monilifera subsp. monilifera in Western Australia
The first confirmed record of C. monilifera subsp. monilifera in Western Australia comes from a specimen collected by Brother Kissane on August 27, 1948, and lodged at the Western Australian Herbarium (PERTH 416444; AVH data). It was nearly three decades, however, before this specimen was formally determined to be C. monilifera subsp. monilifera (Gray Reference Gray1976). The locality of the collection was recorded simply as “Armadale,” but on the same day the collector also lodged a record for beard heath (Leucopogon capitellatus DC.) (PERTH 3004341; AVH data) with a location of “Cooliabberra Spring,” which is either a winter-flowing creek within Bungendore Regional Park off the Albany Highway in Armadale or a private landholding adjacent to the park. Given the temporal proximity, it is very likely that the two records were from a similar area, which would place the first confirmed C. monilifera subsp. monilifera record for Western Australia near a well-known infestation that follows Neerigen Brook alongside the Albany Highway.
The number of residential properties found to have C. monilifera subsp. monilifera in the Perth hills and Narrogin (Batchelor et al. Reference Batchelor, Scott and Webber2024) could suggest that at some point C. monilifera subsp. monilifera was available from plant nurseries. However, the plant has never been listed in any historical Western Australian nursery or seed catalog (J Viska, Australian Garden History Society, personal communication). Cherry (Reference Cherry2010) noted that C. monilifera subsp. monilifera at Dardadine may have been introduced by teachers from South Australia, as this was the site of an old school. Given that the school closed in 1935, this would make that location Western Australia’s earliest plantings, if true. We could not identify any other sources to further clarify the origins of C. monilifera subsp. monilifera in Western Australia, and the origin therefore remains unknown. Molecular studies would be a logical way to provide greater clarity on introduction pathways and timing, which in turn can inform improved management (Emmett et al. Reference Emmett, Scott, Webber, Severn-Ellis and Bell2023).
Ecology and Biology
Genetics
Multiple genetic analyses that included the genus Chrysanthemoides have confirmed that C. monilifera subsp. monilifera is a well circumscribed and separate taxon from C. monilifera subsp. rotunda (Barker et al. Reference Barker, von Senger, Howis, Zachariades and Ripley2005, Reference Barker, Howis, Nordenstam, Källersjö, Eldenäs, Griffioen and Linder2009, Reference Barker, Paterson and Howis2015; Byrne et al. Reference Byrne, Scheben, Scott, Batchelor, Severn-Ellis, Webber, Gooden and Bell2022; Emmett et al. Reference Emmett, Scott, Webber, Severn-Ellis and Bell2023). The distribution of C. monilifera subsp. monilifera in its region of origin is relatively restricted and uncontroversial, occurring in the Western Cape Province of South Africa. There is evidence for C. monilifera subsp. monilifera hybridization with C. monilifera subsp. rotunda in Australia, where the two subspecies’ distributions overlap in north coastal Victoria (Adair and Butler Reference Adair and Butler2010; also herbarium collections MEL 1553294A, CANB 377051.1). There is no record of hybridization in Western Australia. In South Africa, these two Chrysanthemoides monilifera subspecies (i.e., C. monilifera subsp. rotunda and C. monilifera subsp. monilifera) are allopatric (Norlindh Reference Norlindh1943).
Chrysanthemoides monilifera subsp. rotunda is an obligate outcrossing taxon (Gross et al. Reference Gross, Whitehead, de Souza and Mackay2017; Scott et al. Reference Scott, Batchelor and Webber2019b). This means that isolated plants do not produce seeds until another individual germinates nearby and flowers (i.e., subject to Allee effects due to pollination limitations). It is not known if C. monilifera subsp. monilifera is likewise obligately outcrossing, despite the mention of allogamy in Weiss et al. (Reference Weiss, Adair, Edwards, Winkler and Downey2008). This trait needs to be determined, as it has significant implications for the management of C. monilifera subsp. monilifera introductions.
Seed Dispersal by Birds and Mammals
In South Africa, Knight (Reference Knight1988) recorded that 15 bird species visited Chrysanthemoides monilifera plants to feed on fruits, with the most frequent visitors being the sombre bulbul (Andropadus importunus), Cape bulbul (Pycnonotus capensis), and the Rameron pigeon (Columba arquatrix). Knight (Reference Knight1988) did not identify the subspecies of Chrysanthemoides monilifera, but we investigated the flora of his study location (Fernkloof Nature Reserve, Western Cape Province, 34.394°S, 19.265°E) and used its complete flora list with photos (https://www.fernkloof.org.za/index.php/all-plants/plant-families/item/osteospermum-moniliferum-subsp-moniliferum), confirming that the reserve only has C. monilifera subsp. monilifera.
Gosper (Reference Gosper2003) reported on a detailed study of frugivores on C. monilifera subsp. rotunda from coastal New South Wales. Of the 22 bird species feeding on the fruit, the most frequent species were the pied currawong (Strepera graculina), Lewin’s honeyeater (Meliphaga lewinii), silvereye (Zosterops lateralis), red-whiskered bulbul (Pycnonotus jocosus), yellow-faced honeyeater (Lichenostomus chrysops), and olive-backed oriole (Oriolus sagittatus) (Gosper Reference Gosper2003). Emus (Dromaius novaehollandiae) have been reported to consume fruit and may be the vectors responsible for long-distance dispersal (Brougham et al. Reference Brougham, Cherry and Downey2006). While the viability of emu-egested seed has never been confirmed through formal experiments, dense clusters of C. monilifera subsp. monilifera seedlings have been observed germinating from emu scats (Batchelor et al. Reference Batchelor, Scott and Webber2024).
An understanding of seed dispersal potential is critical to management of a bird-dispersed species such as C. monilifera subsp. monilifera. This relationship was studied by Mokotjomela et al. (Reference Mokotjomela, Musil and Esler2013a, Reference Mokotjomela, Musil and Esler2013b, Reference Mokotjomela, Musil and Esler2013c) as part of a broader study examining seed dispersal by frugivores in South Africa. The target plants in the study were two native [C. monilifera subsp. monilifera and African olive (Olea europaea subsp. africana Mill.)] and two non-native plant species [lantana (Lantana camara L.) and wooly nightshade (Solanum mauritianumScopoli)] and the birds were the small species Cape white eye (Zosterops capensis), the medium-size Cape bulbul (Pycnonotus capensis), and the large speckled mousebird (Colius striatus). Flight distances corresponding with predicted seed gut retention times for C. monilifera subsp. monilifera were 9.4 km, 17.8 km, and 21.2 km for Z. capensis, P. capensis, and C. striatus, respectively. These maximum potential distances for seed dispersal, based on theoretical bird-ring recapture frequency and gut retention times, were much greater than that previously reported, which was on the order of 1 km (Mokotjomela et al. Reference Mokotjomela, Musil and Esler2013c). While this study may suggest that very long distance dispersal events are theoretically possible for C. monilifera subsp. monilifera, their likelihood of occurrence in field-relevant conditions is another matter entirely.
Seed Predation by Birds and Mammals
Chrysanthemoides monilifera subsp. monilifera in its native habitat, South Africa, is harvested from plants and from the ground by Chacma baboons (Papio ursinus), but seeds do not survive ingestion (Knight Reference Knight1988). Most seed is not dispersed but falls under the parent plant onto the ground (Knight Reference Knight1988; Scott Reference Scott1996), where seeds are subject to predation by rodents (Scott Reference Scott1996).
In southeastern Australia, Gosper (Reference Gosper2003) recorded C. monilifera subsp. rotunda seed predation by the parrots, crimson rosella (Platycercus elegans) and eastern rosella (Platycercus eximius), and the house sparrow (Passer domesticus). In contrast, C. monilifera subsp. monilifera is not exposed to seed predation by parrots, because the two types of organisms are geographically widely separated in Africa. Chrysanthemoides monilifera subsp. monilifera is restricted to the southwest Western Cape Province, whereas parrots are found in the northern parts of southern Africa (eight species in the family Psittacidae; Newman Reference Newman1983). It is likely that rodent predation is the evolutionary driver producing a “bone” seed (i.e., round and hard).
Seed Germination
Recent seed germination research on C. monilifera subsp. monilifera has focused on germination microclimates and chemical amendments to stimulate germination. Chrysanthemoides monilifera subsp. monilifera seeds germinated faster over a range of temperatures with the application of karrikins to harvested seeds, relative to control treatments (Reynolds et al. Reference Reynolds, Long, Flematti, Cherry and Turner2014). Their study also determined that seed imbibition was rapid (within 48 h) and that dormancy was physiological. Interestingly, germination was not inhibited by the hard, woody endocarp, and dormancy occurred in the winter months. Germination occurred over relatively low temperatures (10 to 20 C), characteristic of winter in southwest Western Australia and ceased at 35 C (Reynolds et al. Reference Reynolds, Long, Flematti, Cherry and Turner2014). In contrast, Batchelor et al. (Reference Batchelor, Yeoh, Bell, Campos, Ota, Richetti and Webber2023b) found that germination of C. monilifera subsp. monilifera seeds was not enhanced by smoke water (containing karrikins), while gibberellic acid accelerated germination but did not increase overall germination percentage. Seeds appeared to have no afterripening phase, and seed desiccation did not increase the likelihood of endocarps fracturing (i.e., to initiate imbibition). Seeds were found to be vulnerable to direct flame exposure, even for short periods of 10 s, suggesting controlled burns may well lead to high mortality for seeds not protected deeper in the soil seedbank (Batchelor et al. Reference Batchelor, Yeoh, Bell, Campos, Ota, Richetti and Webber2023b).
Survival of C. monilifera subsp. monilifera and C. monilifera subsp. rotunda seeds was compared in controlled aging experiments performed at 45 C and 60% relative humidity (Schoeman et al. Reference Schoeman, Buckley, Cherry, Long and Steadman2010). The number of days to lose 50% viability in C. monilifera subsp. monilifera and C. monilifera subsp. rotunda was 47 d and 16 d, respectively. The authors predicted that C. monilifera subsp. monilifera may have a long-lived seedbank and C. monilifera subsp. rotunda a more transient (<1 yr) seedbank (Schoeman et al. Reference Schoeman, Buckley, Cherry, Long and Steadman2010). French et al. (Reference French, Ashcroft, Panetta, Raghu and Cherry2024) buried seeds of C. monilifera subsp. monilifera and C. monilifera subsp. rotunda at two locations and two depths and sampled regularly for 8 yr. Seed viability showed a rapid decline with time, although in excess of 10% of seeds were still alive at 8 yr at some sites. It is possible that edaphic factors, in particular duration of soil moisture, could explain the variation. Detailed field sampling of C. monilifera subsp. rotunda over more than a decade (Scott et al. Reference Scott, Batchelor and Webber2019b) found that the soil seedbank of a non-native population in Kwinana, WA, appeared to have a seed longevity of approximately 7 yr. As the time since last known seed additions to the seedbank increased, it become increasingly difficult to measure seedbank viability, as it consisted of very few seeds (Scott et al. Reference Scott, Batchelor and Webber2019b).
Biotic Resistance
Grazing using goats, sheep, or cattle is good at suppressing C. monilifera subsp. monilifera, but plants soon recovered once the grazers were removed, and there is the risk of seed dispersal via the grazing animals (Briden Reference Briden2008). The Tasmanian distribution of C. monilifera subsp. monilifera is concentrated in cities and towns and is generally absent from the intervening rural areas. Scurr et al. (Reference Scurr, Kirkpatrick, Daniels and McQuillan2008) hypothesized, and demonstrated by exclusion experiments, that grazing by wild macropods (especially Tasmanian pademelons [Thylogale billardierii]) and domesticated herbivores may be sufficient to prevent the spread of C. monilifera subsp. monilifera. This hypothesis could also explain the disparate distribution in Western Australia and needs to be further assessed.
Further supporting the role of native herbivores is the study of overabundant wallabies (mostly swamp wallaby [Wallabia bicolor], plus other grazing marsupials) in the Booderee National Park in southern New South Wales (Dexter et al. Reference Dexter, Hudson, James, MacGregor and Lindenmayer2013). Grazing by wallabies inhibited the recruitment of C. monilifera subsp. rotunda following fire-induced recruitment events in 18 unfenced (browsed) plots, whereas healthy recruitment occurred in 16 fenced (unbrowsed) plots (Dexter et al. Reference Dexter, Hudson, James, MacGregor and Lindenmayer2013).
Allelopathy
Understanding allelopathy is important, as it may have negative impacts on restoration efforts following C. monilifera subsp. monilifera removal. Field experience and anecdotal evidence indicate the likelihood that Chrysanthemoides species have allelopathic effects on other vegetation (Weiss et al. Reference Weiss, Adair, Edwards, Winkler and Downey2008). Vranjic et al. (Reference Vranjic, Woods and Barnard2000) found that shoot and root biomass of coast wattle [Acacia sophorae (Labill.) R. Br.] was significantly lower for seedlings grown in C. monilifera subsp. rotunda soil than for those grown in Acacia soil. Our surveys of C. monilifera subsp. rotunda indicate that seeds were densest under the parent bush, but without germination unless the parent plant was dead for a few years (Scott et al. Reference Scott, Batchelor and Webber2019b). Ens (Reference Ens2007) investigated the allelopathic effects of C. monilifera subsp. rotunda in a Ph.D thesis at Wollongong University and subsequently published that allelopathy in C. monilifera subsp. rotunda was a key mechanism driving the recruitment limitation of indigenous flora (Ens and French Reference Ens and French2008; Ens et al. Reference Ens, French and Bremner2008, Reference Ens, Bremner, French and Korth2009a, Reference Ens, French and Bremner2009b, Reference Ens, French, Bremner and Korth2010).
A second Ph.D on allelopathy in Chrysanthemoides generated a series of papers on C. monilifera subsp. monilifera at Victoria University (Al Harun et al. Reference Al Harun, Robinson, Johnson and Uddin2014, Reference Al Harun, Johnson and Robinson2015a, Reference Al Harun, Johnson, Uddin and Robinson2015b, Reference Al Harun, Johnson, Uddin and Robinson2015c). An initial experiment of aqueous solutions of ground-up C. monilifera subsp. monilifera plant parts (leaves, stem, roots) had no impact on germination of lettuce (Lactuca sativa L., Asteraceae), and little impact on germination of black wattle (Acacia mearnsii De Wild., Fabaceae), but inhibited growth and germination of rock isotome (Isotoma axillaris Lindl., Campanulaceae). The latter two species are found in the Australian environment where C. monilifera subsp. monilifera grows, whereas L. sativa is the usual bioassay for allelopathy studies (Al Harun et al. Reference Al Harun, Robinson, Johnson and Uddin2014).
A test of volatiles coming from C. monilifera subsp. monilifera stems, leaves, and roots, registered no impact on lettuce germination. A similar result occurred with A. mearnsii and strawflower [Xerochrysum bracteatum (Vent.) Tzyelev, Asteraceae] that grow in the same environment as C. monilifera subsp. monilifera (Al Harun et al. Reference Al Harun, Johnson and Robinson2015a). Four phenolic compounds—catechins, p-coumaric acid, phloridzin, and ferulic acid—were identified from C. monilifera subsp. monilifera tissue out of the 13 tested (Al Harun et al. Reference Al Harun, Johnson, Uddin and Robinson2015c). Leachate from C. monilifera subsp. monilifera leaf litter inhibited germination in X. bracteatum and I. axillaris, but unidentified allelochemicals could still be the cause of the allelopathy observed (Al Harun et al. Reference Al Harun, Johnson, Uddin and Robinson2015c). Further experiments using litter alone, litter and soil, and soil alone did not reduce lettuce germination above 20%, indicating an effect (Al Harun et al. Reference Al Harun, Johnson, Uddin and Robinson2015b), albeit marginal in magnitude.
Management Options for Chrysanthemoides monilifera subsp. monilifera
The primary management tools that have been used to control C. monilifera subsp. monilifera infestations in Australia are manual weeding, controlled burns (i.e., fire), and herbicides (Table 1). For dense infestations, mechanical control can be deployed, but unless stumps are removed from the ground, there is a danger that plants will resprout. Classical biocontrol has also been pursued for C. monilifera subsp. monilifera in Australia, including new developments and insight in the last two decades.
Table 1. Summary of methods used to control boneseed (Chrysanthemoides monilifera subsp. monilifera) in Australia a
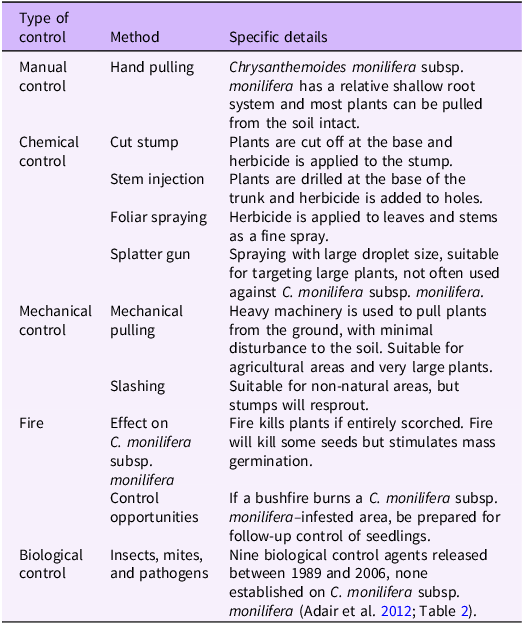
a Adapted from Brougham et al. (Reference Brougham, Cherry and Downey2006) with updates.
Fire
Fire can successfully control C. monilifera subsp. monilifera by killing adult plants and near-surface seeds in the soil. When used as part of an integrated approach (e.g., fire followed by herbicide treatment and/or hand pulling of surviving plants), it is possible to extirpate C. monilifera subsp. monilifera via a controlled burn (i.e., lightly invaded, intact ecosystems; Melland and Preston Reference Melland and Preston2008). Small-scale fire has been applied to C. monilifera subsp. monilifera in Western Australia in the past with good control outcomes (P Hennig, personal communication). For example, at one site in Manypeaks, a small fire over 20 to 30 m2 in 2015 resulted in the emergence of ca. 250 seedlings the following year, which were subsequently controlled, and no plants have been observed since (P Hennig, personal communication). Lindenmayer et al. (Reference Lindenmayer, MacGregor, Dexter, Fortescue and Cochrane2013) demonstrated that a too-frequent fire regime (<5-yr interval) led to the vegetation being dominated by bracken, likely as a result of overgrazing of C. monilifera subsp. rotunda and native plant species by wallabies (Dexter et al. Reference Dexter, Hudson, James, MacGregor and Lindenmayer2013).
Herbicides
Much of the research on optimizing herbicide use on C. monilifera subsp. monilifera was done before 2008 and is summarized in Brougham et al. (Reference Brougham, Cherry and Downey2006) and Weiss et al. (Reference Weiss, Adair, Edwards, Winkler and Downey2008). Little has been published on improving herbicide effectiveness for C. monilifera subsp. monilifera in the last 15 yr, indicating that there is no evidence to show approved herbicides being or becoming ineffective against C. monilifera subsp. monilifera. Herbicide remains an effective control solution for C. monilifera subsp. monilifera, particularly at higher plant densities.
Between 2006 and 2008, when large populations of C. monilifera subsp. monilifera were controlled in Western Australia, herbicide was applied to foliage or by cut-and-paint, with follow-up treatment the following year. Herbicides registered for use on C. monilifera subsp. monilifera in Western Australia include: 2,4-D amine, bromoxynil, glyphosate, metsulfuron, picloram plus 2,4-D, and metsulfuron plus glyphosate (Moore and Moore Reference Moore and Moore2021).
Aerial spraying of herbicide has been used in eastern Australia against C. monilifera subsp. rotunda since 2005 and has been shown to be effective for control of large dense infestations (boom spraying) or individual plants (cone application) (Department of Planning and Environment NSW 2022; Toth and Winkler Reference Toth and Winkler2008). New application mechanisms that allow for targeted application via drones would be worth exploring for C. monilifera subsp. monilifera, as is being practiced for C. monilifera subsp. rotunda (Department of Planning and Environment NSW 2022), particularly if plants are identified in open yet inaccessible areas.
Biological Control
In South Africa, 113 phytophagous arthropods, 3 fungi, and 1 mycoplasm have been found associated with C. monilifera, with 46 taxa (mostly insects) having potential as biological control agents (Scott and Adair Reference Scott and Adair1995; Weiss et al. Reference Weiss, Adair and Edwards1998).
Nine potential agents (arthropod species) have been released in Australia, three potential agents (two insect and one fungus) were studied but not released, and one potential agent was found already established as part of a biological control program for C. monilifera subsp. monilifera and C. monilifera subsp. rotunda (Adair et al. Reference Adair, Morley and Morin2012). Six of these species were approved for release to manage C. monilifera subsp. monilifera: three leaf beetles (Chrysolina spp.), seed fly (Mesoclanis magnipalpis), leaf-roller moth (Tortrix sp.), and leaf buckle mite (Aceria sp.) (Table 2). All except one species (buckle mite) failed to establish on C. monilifera subsp. monilifera, despite multiple releases (Downey et al. Reference Downey, Holtkamp, Ireson, Kwong and Swirepik2007; Morley Reference Morley2010). One agent, the leaf buckle mite, is possibly established in Tasmania (Morley et al. Reference Morley, Ireson and Ivory2012) and Victoria. It was recently observed in 2021 at Mount Eliza, Victoria, at a 2008 release site, but had only spread 25 m (Atlas of Living Australia, Biocontrol Hub; observation ID TM210214_01; https://biocollect.ala.org.au/biocontrolhub).
Table 2. Biological control releases on boneseed (Chrysanthemoides monilifera subsp. monilifera) in Australia a
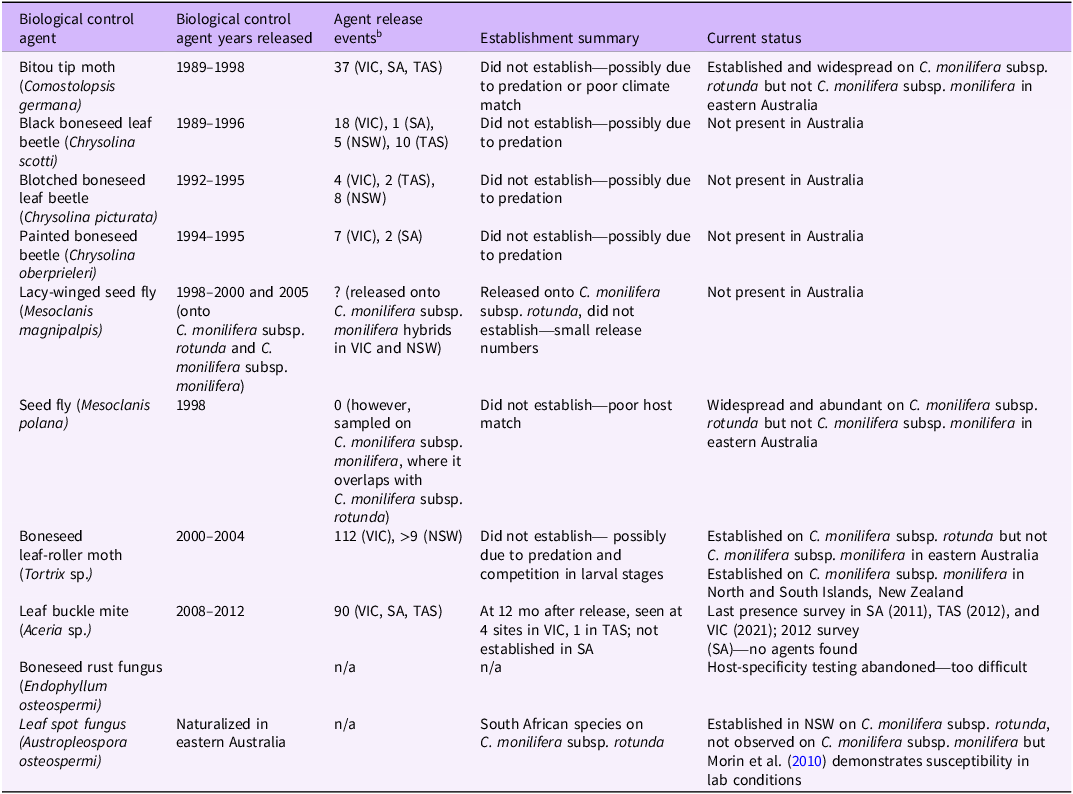
a Adapted from Brougham et al. (Reference Brougham, Cherry and Downey2006) and Adair et al. (Reference Adair, Morley and Morin2012) with updates.
b NSW, New South Wales; SA, South Australia; TAS, Tasmania; VIC, Victoria.
The release of Tortrix sp. in New Zealand to target introduced C. monilifera subsp. monilifera was initially thought to be unsuccessful, with only “patchy” establishment (Paynter et al. Reference Paynter, Forgie, Winks, Peterson, Ward, Nicholson and Van Zoelen2012) on the North Island and failure to establish on the South Island (Bownes Reference Bownes2022). By 2022, 11 yr postrelease, establishment was reported for the South Island, with indications of damage caused by the biological control agent (Bownes Reference Bownes2022).
An application to release boneseed rust (Endophyllum osteospermi) was not made due to the 2-yr life cycle making it extremely difficult to do testing and get the necessary supporting data (L Morin, personal communication). Another pathogen, Austropleospora osteospermi (syn.: Hendersonia osteospermi), is a leaf spot that somehow naturalized in Australia from southern Africa and is now widespread on C. monilifera subsp. rotunda in coastal New South Wales (Morin et al. Reference Morin, Shivas, Piper and Tan2010) but with no (or limited) demonstrated impact. Its host range is limited to Chrysanthemoides and close relatives, so it could be considered for use as a biological control agent against C. monilifera subsp. monilifera in Western Australia (Morin et al. Reference Morin, Shivas, Piper and Tan2010), but its absence from the state needs to first be confirmed.
As of 2012, there were no agents being actively researched in Australia (Adair et al. Reference Adair, Morley and Morin2012). A lack of resourcing has resulted in no further C. monilifera subsp. monilifera biocontrol agent development since that time. The obvious question is why so many biocontrol agents have been worked up to a stage where they were approved for release, but have subsequently failed to establish on C. monilifera subsp. monilifera. Predation by native invertebrates appears to have hampered establishment or dispersal of Chrysolina spp. and Tortrix sp. (Adair et al. Reference Adair, Morley and Morin2012). Other reasons could be a poor climate match or poor genetic match as part of the host-matching process.
We now have a comprehensive knowledge of the genetics of the closely related C. monilifera subsp. rotunda, based on nuclear and chloroplast genomes, which has enabled us to identify the source population in South Africa for the single introduction(s) to Australia (Emmett et al. Reference Emmett, Scott, Webber, Severn-Ellis and Bell2023). This work has identified a previous unsearched region in South Africa where potentially more suitable or specific biological control agents could be found. In contrast, C. monilifera subsp. monilifera has not been studied in relation to genetic variation or pollination syndromes, with only some glimpses of the genome where C. monilifera subsp. monilifera has been used as the outlier group in the study of C. monilifera subsp. rotunda.
An improvement in host–agent matching would be a possible outcome from such molecular work. All past agents were reared on Australian C. monilifera subsp. monilifera plants while in quarantine. However, the nutrient and physical status of plants in the field may have been very different from those used for agent rearing and testing in quarantine (e.g., not fertilized, greater leaf toughness). It seems unlikely that climate mismatch between invaded areas and regions where agent searching was conducted has played a role in the lack of success, at least in southeastern Australia. However, current climate-matching models (Adair et al. Reference Adair, Morley and Morin2012) are inadequate, as projections extend implausibly into desert areas that are unlikely to be suitable for C. monilifera subsp. monilifera. If further agent searching was considered, it would be a priority to develop more robust process-based models to inform the agent development pipeline (Kriticos et al. Reference Kriticos, Ireland, Morin, Kumaran, Rafter, Ota and Raghu2021).
Integrated Control
Recent work on delivering more effective management outcomes for C. monilifera subsp. rotunda control has coalesced around a management approach involving spraying with herbicide, burning, then respraying (Lindenmayer et al. Reference Lindenmayer, Wood, MacGregor, Buckley, Dexter, Fortescue, Hobbs and Catford2015; O’Loughlin et al. Reference O’Loughlin, Gooden, Foster, MacGregor, Catford and Lindenmayer2019). A similar approach is recommended to eliminate C. monilifera subsp. monilifera (Melland Reference Melland2007; Melland and Preston Reference Melland and Preston2008; Melland et al. Reference Melland, Ainsworth and Roush1999). At 6 to 12 mo before a fire, the infestation should be prepared by hand pulling and cutting C. monilifera subsp. monilifera, so as to provide fuel at ground level, otherwise a fire might be too patchy (Melland et al. Reference Melland, Ainsworth and Roush1999).
Fire in autumn at 250 to 300 C will kill C. monilifera subsp. monilifera plants and deplete the seedbank (Brougham et al. Reference Brougham, Cherry and Downey2006). Management of C. monilifera subsp. monilifera is achieved when followed in 18 mo by herbicide treatment or hand pulling of surviving plants or new germinants. This approach works best at a density of under 1,000 seeds m–2 (i.e., a light infestation). For heavier seedbank densities (>1,000 seeds m–2), fire treatments are only likely to produce lower population abundance rather than localized extirpation (Melland Reference Melland2007; Melland and Preston Reference Melland and Preston2008; Melland et al. Reference Melland, Ainsworth and Roush1999).
Detection
For management programs that are targeting localized extirpation or containment, improving detection of infestations and single plants for subsequent control is a priority. The role of citizen science in documenting new infestations, particularly via online reporting platforms (Howard et al. Reference Howard, van Rees, Dahlquist, Luikart and Hand2022), has increased significantly in the last two decades (e.g., Landscape South Australia 2020). Given that much of the control of C. monilifera subsp. monilifera across Australia is undertaken by community groups, online apps can be very effective tools.
New technologies have been applied to C. monilifera subsp. monilifera eradication programs to address the challenges of detecting rare plants in landscapes where operation is difficult due to access or terrain. Honey bee (Apis mellifera) hives were tested as potential aggregation tool for environmental DNA (eDNA; pollen in this case) to detect C. monilifera subsp. monilifera plants in an urban landscape (Batchelor et al. Reference Batchelor, Bell, Campos and Webber2023a). A species-specific assay was developed and a proof-of-concept trial was successful using pollen collected from bees foraging in dense C. monilifera subsp. monilifera populations in South Australia. However, no C. monilifera subsp. monilifera DNA was detected when the method was applied to material from hive pollen traps situated near isolated C. monilifera subsp. monilifera plants in Western Australia (Batchelor et al. Reference Batchelor, Bell, Campos and Webber2023a). It may be that other eDNA substrates (Bell et al. Reference Bell, Campos, Hoffmann, Encinas-Viso, Hunter and Webber2024) could be developed for C. monilifera subsp. monilifera using the same PCR assay.
Detection of isolated plants in heterogeneous landscapes is also being widely addressed using drone-based remote-sensing methods. These methods were applied to detecting low-density C. monilifera subsp. monilifera plants in Western Australia with mixed success (Batchelor et al. Reference Batchelor, Yeoh, Bell, Campos, Ota, Richetti and Webber2023b). Both visible and multispectral band imagery were stitched into an orthomosaic for subsequent image classification based on pixel reflectance values. The models ended up with high performance metrics but unacceptably high type II error, a likely artifact of insufficient training imagery from Western Australia to develop the model (images from high-density C. monilifera subsp. monilifera populations in South Australia were used, which likely created model transferability issues; Batchelor et al. Reference Batchelor, Yeoh, Bell, Campos, Ota, Richetti and Webber2023b). Similar drone-based methods for plant detection have recently been developed for C. monilifera subsp. rotunda (Amarasingam et al. Reference Amarasingam, Kelly, Sandino, Hamilton, Gonzalez, Dehaan, Zheng and Cherry2024). There may be learning opportunities to adapt this model for C. monilifera subsp. monilifera. However, habitat differences mean that C. monilifera subsp. monilifera is frequently found in environments with a tree overstory (as opposed to the often-open dune environments occupied by introduced C. monilifera subsp. rotunda), making the issue of image processing and analysis (i.e., orthomosaic vs. single-image workflows) an important consideration.
Lessons Learned from Management Elsewhere
Management in Southeastern Australia
While Western Australia has been in the enviable position of being able to maintain an eradication goal for C. monilifera subsp. monilifera across the entire state, Victoria, South Australia, and Tasmania (where C. monilifera subsp. monilifera is abundant) are mostly focusing on containment or asset protection. In 2014, the Eastern Australia Boneseed Eradication Project was working across southern and western New South Wales and eastern Victoria to eradicate all known infestations of C. monilifera subsp. monilifera from the former state and seeking to establish a new national containment line on the New South Wales border in a strategic, cross-jurisdictional effort (Martin Reference Martin2013). This effort, combined with the South Coast Bitou Bush Task Force and relevant stakeholders, implemented the national southern C. monilifera subsp. rotunda containment line at Tuross Head, NSW, to prevent C. monilifera subsp. rotunda from spreading south and hybridizing with C. monilifera subsp. monilifera spreading up from Victoria (Cherry Reference Cherry2010; Cherry et al. Reference Cherry, Leighton, Clark and Austin2006).
South Australia has a declared plant policy for C. monilifera subsp. monilifera under the Landscape South Australia Act 2019, which as of 2021 aimed to eradicate C. monilifera subsp. monilifera from the Northern, Yorke, and Eyre Peninsula and South Australian Arid Lands, contain C. monilifera subsp. monilifera in the Murraylands and Riverland and Limestone Coast (via destruction of outlier populations and the Murray-Coorong Boneseed Containment Zone), and asset protect in the Hills and Fleurieu and Green Adelaide areas. More recently, it has been accepted that it will not be feasible to eradicate C. monilifera subsp. monilifera from the Northern and Yorke region, which has moved to a contain approach (D Hughes, Landscape South Australia, personal communication). The Kangaroo Island and Alintjara Wiluranara regions are still free of C. monilifera subsp. monilifera (Government of South Australia 2021; J Walter, Landscape South Australia, personal communication).
In Tasmania, C. monilifera subsp. monilifera is a declared weed under the Biosecurity Act 2019. Eradication remains the stated aim in the northwest, where all C. monilifera subsp. monilifera has been controlled and local land managers are engaged (Cherry Reference Cherry2010; Department of Natural Resources and Environment Tasmania 2011). In the south and northeast, all outlier populations are listed as control priorities, and containment lines are maintained around core infestations to prevent further spread to areas known to be free (or in the process of becoming free) of C. monilifera subsp. monilifera (Cherry Reference Cherry2010; Department of Natural Resources and Environment Tasmania 2011). We note that these plans are now more than a decade old and we were not able to confirm that an active coordinated program is still in place.
Chrysanthemoides monilifera subsp. monilifera is listed as a noxious weed (Schedule 2) in Victoria under the Catchment and Land Protections Act 1994 and was identified as an environmental weed with typically significant impacts and a “high” risk rating by White et al. (Reference White, Cheal, Carr, Adair, Blood and Meagher2018). In Victoria, there is regional level variation as to whether the weed is regionally prohibited or regionally controlled. What action is taken is often undertaken by community Landcare groups. Currently, C. monilifera subsp. monilifera is listed for local eradication in the East Gippsland, North Central, and North East regions, whereas the weed is managed by prevention of growth and spread, particularly around high-value conservation areas, in the Corangamite, Glenelg Hopkins, Goulburn, Port Phillip and Westernport, and West Gippsland regions. Control in the Wimmera and Mallee regions was initially focused on eradication but has now transitioned to regional control only. At Port Phillip and Westernport, there appear to be ongoing eradication efforts and asset protection targeting a hybrid C. monilifera subsp. rotunda–C. monilifera subsp. monilifera population.
Across all southeastern states in Australia, the methods being deployed for C. monilifera subsp. monilifera management remain consistent with those outlined in previous guidelines (e.g., Brougham et al. Reference Brougham, Cherry and Downey2006). The failure of classical biological control agents to establish in Australia has meant that manual control, herbicide treatments, managed burns, and occasionally mechanical control are the dominant approaches deployed, often in combination.
We were unable to confirm any records of C. monilifera subsp. monilifera from Norfolk Island, a small Australian territory in the Pacific Ocean, as reported by Mariotti and Zappa (Reference Mariotti and Zappa2022). Rather, this report appears to be a misidentification of C. monilifera subsp. rotunda, which was recently noted as a new record for the remote island (Martoni et al. Reference Martoni, Achari, Blacket, Brohier, Constable, Dugdale, Ekanayake, Kant, Kelly, Kinoti, Li, Lovelock, Mann, Nogarotto, Sawbridge and Smith2023). The infestation was first documented in 2011 under a large Norfolk Island pine tree [Araucaria heterophylla (Sailsb.) Franco] and spread from a small area (ca. 2 m2) to cover more than 100 m2 at its peak in around 2021 (T Patel, Norfolk Island Regional Council, personal communication). The infestation has been actively managed in recent years with the main infestation mechanically mulched and individual plants that had established on the nearby coastal cliffs being hand weeded (T Patel, Norfolk Island Regional Council, personal communication). Learning from C. monilifera subsp. rotunda eradications in Western Australia (Scott et al. Reference Scott, Batchelor, Jucker and Webber2019a) and Queensland (Cherry et al. Reference Cherry, Willsher and Whyte2008) is of clear relevance for a successful eradication of C. monilifera subsp. rotunda on Norfolk Island.
Management in New Zealand
Chrysanthemoides monilifera subsp. monilifera is also a non-native invasive weed in New Zealand. It was first recorded in 1870 and after a very long lag phase became prominent in the 1990s (Briden Reference Briden2008). Invasion initially was localized to urban areas, but now occurs in a wide range of native vegetation and situations from dunes to islands (Briden Reference Briden2008). The strategy in New Zealand is to have surveillance and weed-led control to find and eradicate new infestations and a site-led approach to manage large infestations in valuable ecosystems.
The range of control methods—manual, herbicide, mechanical, and biological control—used in New Zealand are similar to the controls outlined in the Boneseed Management Manual (Brougham et al. Reference Brougham, Cherry and Downey2006; Table 1). An additional control method used in New Zealand is the “mechanical shredder,” a mechanical mulcher mounted in an all-terrain vehicle with rubber tracks (Briden Reference Briden2008). Biological control activities have recently been revived, with the redistribution of Tortrix sp. encouraged now that it is established in the South Island (Bownes Reference Bownes2022).
The New Zealand experience is that the effort required for control decreases over time. Most effort is required in the first 1 to 3 yr with the removal of large plants and control of seedlings. After 5 to 6 yr, the ongoing seedbank is much reduced, and ongoing maintenance takes little effort (Briden Reference Briden2008). However, this approach does not consider the impact of fire. A C. monilifera subsp. monilifera infestation cleared 8.5 yr previously had a massive germination of seeds from the soil seedbank following a fire (Briden and McAlpine Reference Briden and McAlpine2012). There is also evidence that soil disturbance and canopy removal stimulate germination of C. monilifera subsp. monilifera seeds over native plants, indicating that any form of disturbance to an ecosystem will favor germination of C. monilifera subsp. monilifera (McAlpine et al. Reference McAlpine, Timmins and Westbrooke2009). Howell (Reference Howell2012) assessed 10 yr of progress towards environmental weed eradication in New Zealand. His sample of 90 eradication programs included 2 on C. monilifera subsp. monilifera that had made zero progress. The main conclusion of this work was that to succeed with any weed eradication, it pays to start few programs and to focus on those most likely to succeed.
Management Elsewhere (Chile, United States, and Europe)
Atala et al. (Reference Atala, Reyes, Osses, Jeldes-Cajas and Vargas2023) describe the impact on local species diversity of C. monilifera in Valparaíso, Chile, without identifying the subspecies involved. Numerous photos are available online of the study area showing flowers and fruits (Fundación RA Philippi de Estudios Naturales 2024), which enables us to conclude that the subspecies is most likely C. monilifera subsp. monilifera. The invasion is attributed to the introduction as an ornamental plant to the Quinta Vergara Park in Viña del Mar, Chile, from which it escaped cultivation and spread to inland dunes of the Valparaíso region (Atala et al. Reference Atala, Reyes, Osses, Jeldes-Cajas and Vargas2023). We could not find any evidence of management for this invasion and would suggest that further work to confirm identification would be prudent.
Similarly, there does not appear to be active management of C. monilifera subsp. monilifera in California, USA, where it is cultivated as a garden plant and naturalized, but reportedly infrequently escapes and/or rarely persists in the flora (Brusati et al. Reference Brusati, Johnson and DiTomaso2014; Strother Reference Strother2006).
Chrysanthemoides monilifera subsp. monilifera is recorded from multiple areas across Europe, including France (Channel Islands, Saint Raphaël, Théoule sur Mer) Monaco, Italy (Sicily, Ventimiglia), Gibraltar, and Andorra (Bock Reference Bock2024; Greuter Reference Greuter2006; Mariotti and Zappa Reference Mariotti and Zappa2022). We were not able to obtain independent verification of the report from Andorra. This location, given its high altitude and winter climate, seems an unlikely spot for C. monilifera subsp. monilifera to establish. In Italy, Mariotti and Zappa (Reference Mariotti and Zappa2022) report that the Sicilian population of C. monilifera subsp. monilifera has been eradicated and that the Ventimiglia introduction took place in 1869 via seeds planted at the Hanbury Botanic Gardens. The Ventimiglia population was reported as naturalized in 1996 as part of the first survey to assess the status of non-native plants of the area.
Occasional reports of “boneseed” being introduced to Saint Helena, a remote island in the South Atlantic Ocean, are likely a misreported occurrence based on common name confusions. The closely related St. Helena boneseed (Osteospermum sanctae-helenae Norl.), an endemic to Saint Helena, is also sometimes referred to as simply ‘boneseed’ (Cronk Reference Cronk1987).
Updated Insight to Guide Chrysanthemoides monilifera subsp. monilifera Management
In the nearly four decades since C. monilifera subsp. monilifera was first targeted for control in Western Australia, management outcomes have had mixed success. Existing ecological knowledge has generally been sufficient to guide effective management choices, particularly with sparsely distributed plants. However, if eradication is going to be achieved in the state, a step change in management will be required over a considerable duration. The recent assessment of past management and current distribution (Batchelor et al. Reference Batchelor, Scott and Webber2024) provides a robust platform for launching a management program in Western Australia. This review has delivered additional complementary insight across four areas that could help to further improve the program in Western Australia, as well as C. monilifera subsp. monilifera management programs elsewhere.
First, the origins and introduction pathway for C. monilifera subsp. monilifera in Western Australia remain unclear. Improving understanding in this regard, particularly if combined with insight across the full Australian and South African C. monilifera subsp. monilifera distribution, would help to guide genetically informed native range surveys for classical biological control agents. It is important to note that classical biological control remains an unfeasible management option for Western Australia, particularly given the lack of any notable effectiveness elsewhere and the difficulty of maintaining agent presence with the very low ongoing abundance of mature plants. More generally, however, the recently reported positive impacts of Tortrix on New Zealand C. monilifera subsp. monilifera suggest there may be benefit in reevaluating this agent failure elsewhere where containment or minimizing impact is a management goal (e.g., southeastern Australia).
Second, improved knowledge on the ecology and biology of C. monilifera subsp. monilifera has reinforced that currently deployed control methods remain the most effective for use in an eradication program for Western Australia. Important knowledge gaps remain with respect to determining whether C. monilifera subsp. monilifera is capable of self-fertilization (autogamy), and the full cohort of dispersal agents across the introduced range. Both emus and the currently controlled non-native starlings (Sturnus vulgaris) could challenge the working assumption that a 500-m buffer zone (based on dispersal kernels) is adequate to inform population delimitation in Western Australia (Batchelor et al. Reference Batchelor, Scott and Webber2024). Some ecological knowledge gaps have also been addressed to provide management insight. Failure to completely extinguish the soil seedbank remains the biggest threat to a successful eradication campaign (Panetta Reference Panetta2004). Ten years would be a minimum time frame to actively manage a C. monilifera subsp. monilifera seedbank, with 15 yr as a more conservative duration likely necessary for eradication programs. Evidence for using chemical amendments to accelerate seedbank depletion is inconclusive, yet fire appears to cause significant seed mortality.
Third, insight from active programs suggests that a combination of manual, chemical, and fire-based control, with mechanical removal for large infestations, is still optimal for C. monilifera subsp. monilifera management in Western Australia. This knowledge could also be applied to overseas introductions where active management seems absent. An opportunity to eradicate C. monilifera subsp. monilifera before it has spread widely, particularly in areas with Mediterranean climates, appears to be worth prioritizing, even if confirmed identifications found the introduction to be another taxon of non-native Chrysanthemoides.
Finally, novel detection techniques for isolated plants in heterogenous or hard to access landscapes could transform the likelihood of achieving successful eradication of C. monilifera subsp. monilifera in Western Australia. Such techniques are equally applicable to maintaining containment lines for introduced C. monilifera subsp. monilifera elsewhere. While initial attempts to develop eDNA and drone-based remote-sensing methods to detect C. monilifera subsp. monilifera were not successful, they generated promising insights that could be further refined in future work.
This synthesis from invasions in Australia’s southeastern states and elsewhere overseas, combined with updated ecological knowledge of the local weed context, suggests that C. monilifera subsp. monilifera continues to represent a significant weed threat to natural ecosystems in Western Australia. Integrating knowledge obtained from this literature review with a collated baseline of past management efforts (Batchelor et al. Reference Batchelor, Scott and Webber2024) to produce robust and enduring management programs will give land managers the best chance of achieving their eradication objectives.
Funding statement
This work was funded by CSIRO and DPIRD’s Boosting Biosecurity Defences program and made possible by the Royalties for Regions program.
Competing interests
No competing interests have been declared.





