No CrossRef data available.
Article contents
Dynamics of rotating helices in a viscous fluid
Published online by Cambridge University Press: 13 June 2025
Abstract
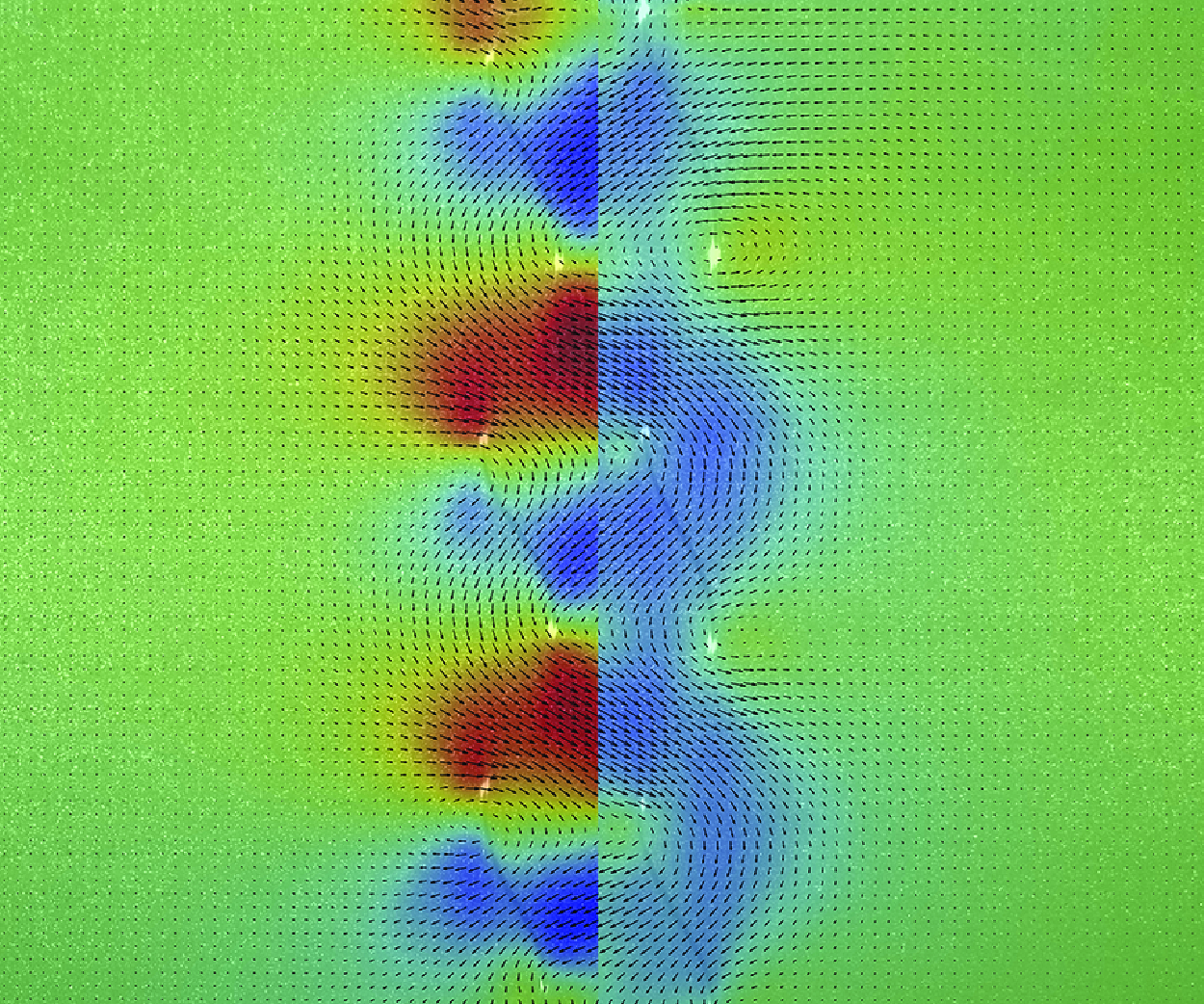
We investigate the dynamics of a pair of rigid rotating helices in a viscous fluid, as a model for bacterial flagellar bundle and a prototype of microfluidic pumps. Combining experiments with hydrodynamic modelling, we examine how spacing and phase difference between the two helices affect their torque, flow field and fluid transport capacity at low Reynolds numbers. Hydrodynamic coupling reduces the torque when the helices rotate in phase at constant angular speed, but increases the torque when they rotate out of phase. We identify a critical phase difference, at which the hydrodynamic coupling vanishes despite the close spacing between the helices. A simple model, based on the flow characteristics and positioning of a single helix, is constructed, which quantitatively predicts the torque of the helical pair in both unbounded and confined systems. Finally, we show the influence of spacing and phase difference on the axial flux and the pump efficiency of the helices. Our findings shed light on the function of bacterial flagella and provide design principles for efficient low-Reynolds-number pumps.
Information
- Type
- JFM Rapids
- Information
- Copyright
- © The Author(s), 2025. Published by Cambridge University Press


